Question
complete the table Standardization of NaOH Dry Lab Data (Titratior Trial 1 Trial 2 0.3327 0.3291 0.1025 0.1025 0.49 1.22 1.22 1.88 0.03 13.15 13.15
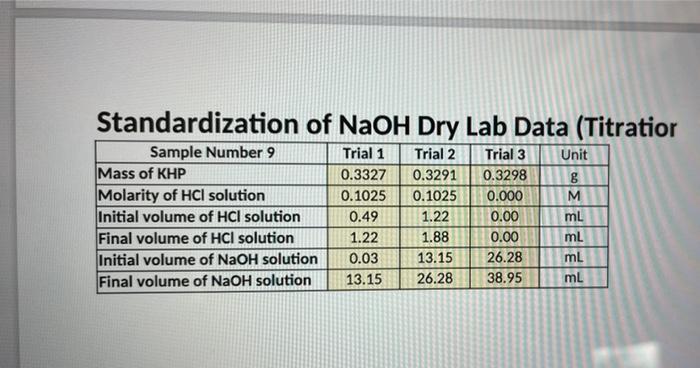
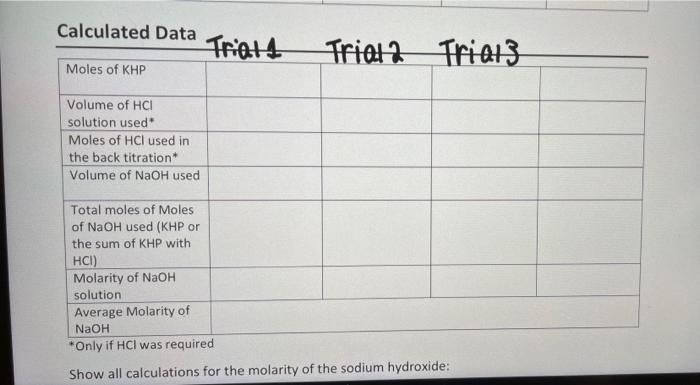
Standardization of NaOH Dry Lab Data (Titratior Trial 1 Trial 2 0.3327 0.3291 0.1025 0.1025 0.49 1.22 1.22 1.88 0.03 13.15 13.15 26.28 Sample Number 9 Mass of KHP Molarity of HCI solution Initial volume of HCI solution Final volume of HCI solution Initial volume of NaOH solution Final volume of NaOH solution Trial 3 0.3298 0.000 0.00 0.00 26.28 38.95 Unit g M mL mL mL mL
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To complete the table we need to calculate the missing values based on the given data Lets start by ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Understanding Basic Statistics
Authors: Charles Henry Brase, Corrinne Pellillo Brase
6th Edition
978-1133525097, 1133525091, 1111827028, 978-1133110316, 1133110312, 978-1111827021
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



