Question
Compound A has a larger enthalpy (heat) of condensation than compound B. Which of the following statements is true? i. Heat will be released
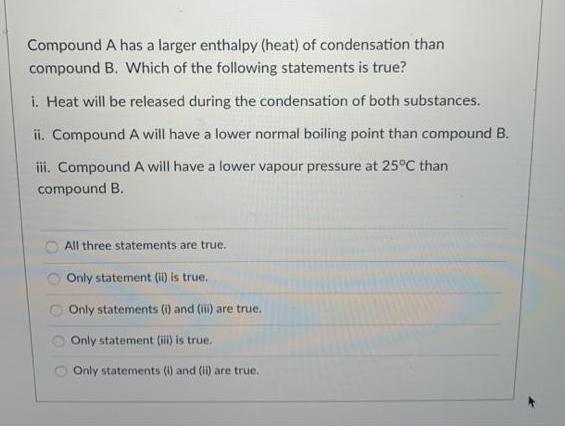
Compound A has a larger enthalpy (heat) of condensation than compound B. Which of the following statements is true? i. Heat will be released during the condensation of both substances. ii. Compound A will have a lower normal boiling point than compound B. ii. CompoundA will have a lower vapour pressure at 25C than compound B. All three statements are true. Only statement (ii) is true. Only statements (i) and (ii) are true. Only statement (ili) is true. Only statements (i) and (i) are true.
Step by Step Solution
3.29 Rating (143 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Accounting concepts and applications
Authors: Albrecht Stice, Stice Swain
11th Edition
978-0538750196, 538745487, 538750197, 978-0538745482
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



