Answered step by step
Verified Expert Solution
Question
1 Approved Answer
compound: C6H5Cl 2. Rewrite the balanced chemical equation from Part A, drawing the Lewis structures of all the compounds involved instead of just writing the
compound: C6H5Cl 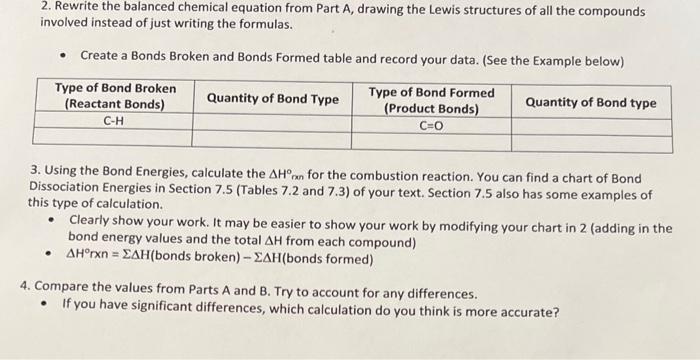
2. Rewrite the balanced chemical equation from Part A, drawing the Lewis structures of all the compounds involved instead of just writing the formulas. - Create a Bonds Broken and Bonds Formed table and record your data. (See the Example below) 3. Using the Bond Energies, calculate the Hinn for the combustion reaction. You can find a chart of Bond Dissociation Energies in Section 7.5 (Tables 7.2 and 7.3 ) of your text. Section 7.5 also has some examples of this type of calculation. - Clearly show your work. It may be easier to show your work by modifying your chart in 2 (adding in the bond energy values and the total H from each compound) - Hrxn=H (bonds broken) H (bonds formed) 4. Compare the values from Parts A and B. Try to account for any differences. - If you have significant differences, which calculation do you think is more accurate 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


