Answered step by step
Verified Expert Solution
Question
1 Approved Answer
concentr 4.16 Consider the following chain-reaction mechanism for the high-temperat formation of nitric oxide, i.e... the Zeldovich mechanism: O+N, NO+N Reaction I N+O, NO+O

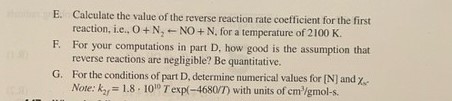
concentr 4.16 Consider the following chain-reaction mechanism for the high-temperat formation of nitric oxide, i.e... the Zeldovich mechanism: O+N, NO+N Reaction I N+O, NO+O Reaction 2 A. Write out expressions for d[NO]/dr and d[N]/dr. B. Assuming N atoms exist in steady state and that the concentrations of O, O., and N, are at their equilibrium values for a specified ten perature and composition, simplify your expression obtained above for d[NO]/dr for the case of negligible reverse reactions. (Aner d[NO]/dt = 2k,[0],[N]) C. Write out the expression for the steady-state N-atom concentration used in part B. D. For the conditions given below and using the assumptions of part how long does it take to form 50 ppm (mole fraction - 10") of NO T=2100 K. p=0.167 kg/m, MW 28.778 kg/kmol, Xo 7.6-10 (mole fraction), 3.025-10-(mole fraction). Xo, Xs, 0.726 (mole fraction). ky 1.82 101 expl-38,370/7(K)] with units of cm/gmol-s E. Calculate the value of the reverse reaction rate coefficient for the first reaction, i.e., O+N, NO + N. for a temperature of 2100 K. F. For your computations in part D, how good is the assumption that reverse reactions are negligible? Be quantitative. G. For the conditions of part D, determine numerical values for [N] and X- Note: k 1.8 100 T exp(-4680/7) with units of cm/gmol-s. =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


