Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a 6s orbital in a hydrogen atom. How many orbitals of this type are possible? Number (Enter an Integer.) How many radial nodes
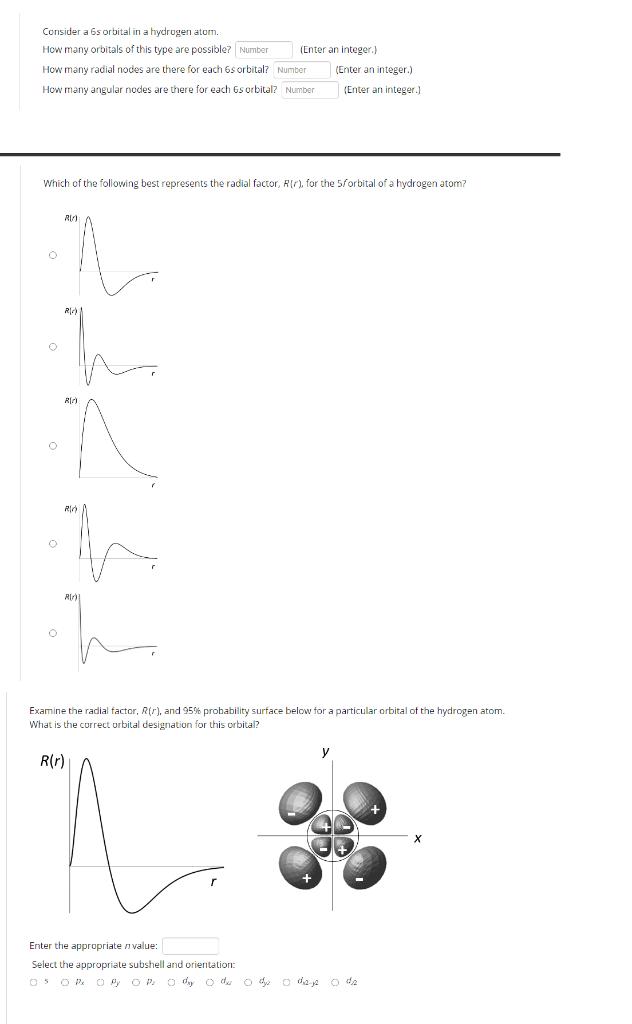
Consider a 6s orbital in a hydrogen atom. How many orbitals of this type are possible? Number (Enter an Integer.) How many radial nodes are there for each 6s orbital? Number How many angular nodes are there for each 6s orbital? Number (Enter an integer.) (Enter an integer.) Which of the following best represents the radial factor, R(r), for the 5/orbital of a hydrogen atom? R R R) R) Examine the radial factor, R(r), and 95% probability surface below for a particular orbital of the hydrogen atom. What is the correct orbital designation for this orbital? R(r) Enter the appropriate value: Select the appropriate subshell and orientation: 05 O Px O P O p o day o du o dy o daje o dz X
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


