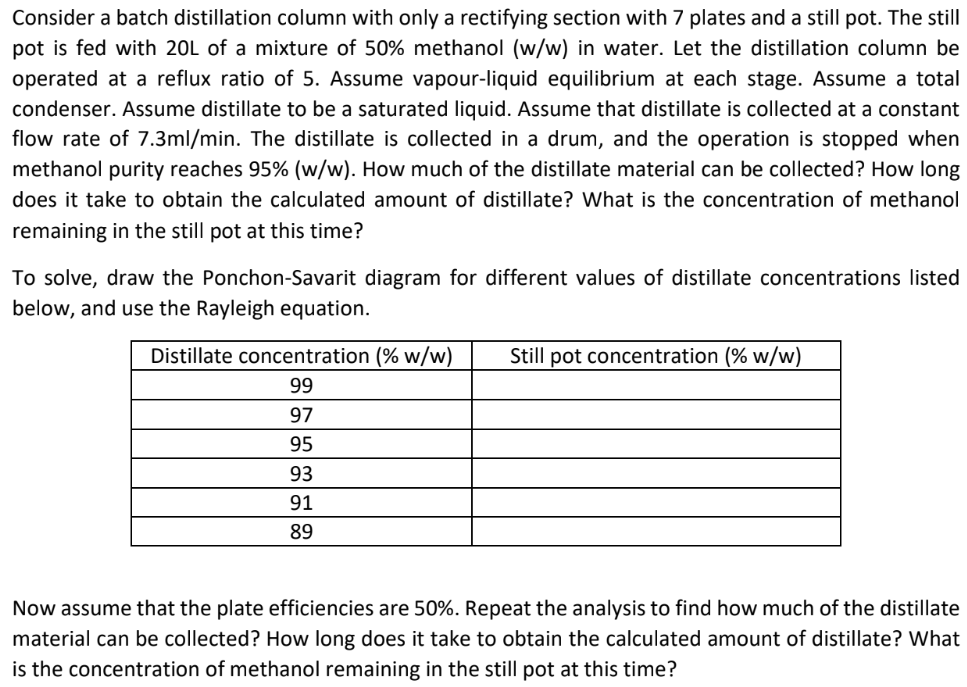
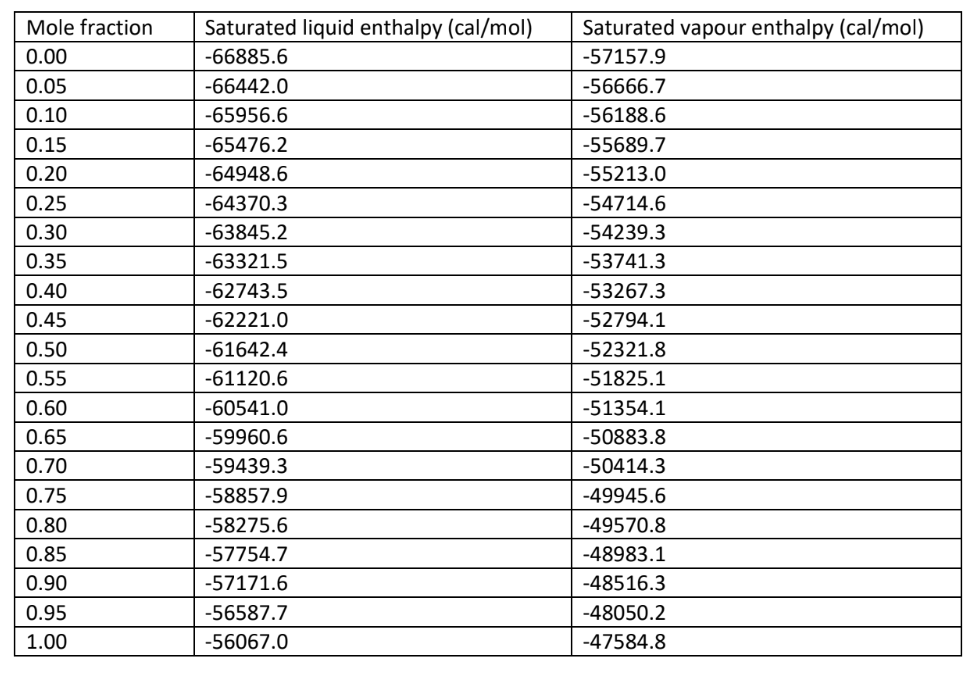
Consider a batch distillation column with only a rectifying section with 7 plates and a still pot. The still pot is fed with 20L of a mixture of 50% methanol (w/w) in water. Let the distillation column be operated at a reflux ratio of 5 . Assume vapour-liquid equilibrium at each stage. Assume a total condenser. Assume distillate to be a saturated liquid. Assume that distillate is collected at a constant flow rate of 7.3ml/min. The distillate is collected in a drum, and the operation is stopped when methanol purity reaches 95%(w/w). How much of the distillate material can be collected? How long does it take to obtain the calculated amount of distillate? What is the concentration of methanol remaining in the still pot at this time? To solve, draw the Ponchon-Savarit diagram for different values of distillate concentrations listed below, and use the Rayleigh equation. Now assume that the plate efficiencies are 50\%. Repeat the analysis to find how much of the distillate material can be collected? How long does it take to obtain the calculated amount of distillate? What is the concentration of methanol remaining in the still pot at this time? \begin{tabular}{|l|l|l|} \hline Mole fraction & Saturated liquid enthalpy (cal/mol) & Saturated vapour enthalpy (cal/mol) \\ \hline 0.00 & -66885.6 & -57157.9 \\ \hline 0.05 & -66442.0 & -56666.7 \\ \hline 0.10 & -65956.6 & -56188.6 \\ \hline 0.15 & -65476.2 & -55689.7 \\ \hline 0.20 & -64948.6 & -55213.0 \\ \hline 0.25 & -64370.3 & -54714.6 \\ \hline 0.30 & -63845.2 & -54239.3 \\ \hline 0.35 & -63321.5 & -53741.3 \\ \hline 0.40 & -62743.5 & -53267.3 \\ \hline 0.45 & -62221.0 & -52794.1 \\ \hline 0.50 & -61642.4 & -52321.8 \\ \hline 0.55 & -61120.6 & -51825.1 \\ \hline 0.60 & -60541.0 & -51354.1 \\ \hline 0.65 & -59960.6 & -50883.8 \\ \hline 0.70 & -59439.3 & -50414.3 \\ \hline 0.75 & -58857.9 & -49945.6 \\ \hline 0.80 & -58275.6 & -49570.8 \\ \hline 0.85 & -57754.7 & -48983.1 \\ \hline 0.90 & -57171.6 & -48516.3 \\ \hline 0.95 & -56587.7 & -48050.2 \\ \hline 1.00 & -56067.0 & -47584.8 \\ \hline \end{tabular}








