Question
Consider a concentrated hydrochloric acid with the following specifications: 37.0 % (w/w) HCI density of the reagent is 1.19 g/mL Express the concentration 37.0%
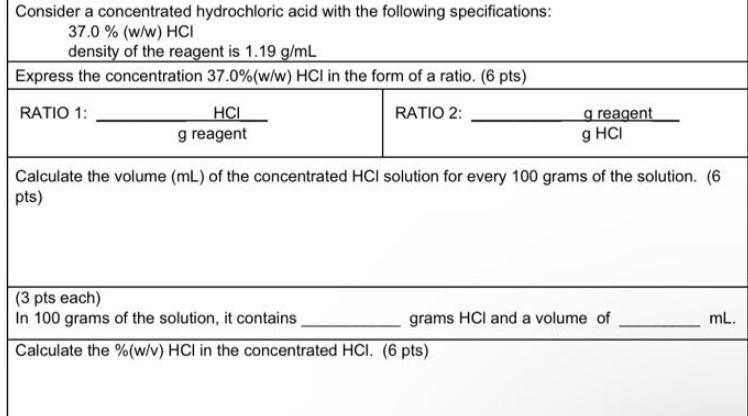
Consider a concentrated hydrochloric acid with the following specifications: 37.0 % (w/w) HCI density of the reagent is 1.19 g/mL Express the concentration 37.0% (w/w) HCI in the form of a ratio. (6 pts) RATIO 1: RATIO 2: HCI g reagent g reagent g HCI Calculate the volume (mL) of the concentrated HCI solution for every 100 grams of the solution. (6 pts) (3 pts each) In 100 grams of the solution, it contains Calculate the % (w/v) HCI in the concentrated HCI. (6 pts) grams HCI and a volume of mL.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
RATIO 1 HCI 370 g HCI g reagent 100 g reagent ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



