Answered step by step
Verified Expert Solution
Question
1 Approved Answer
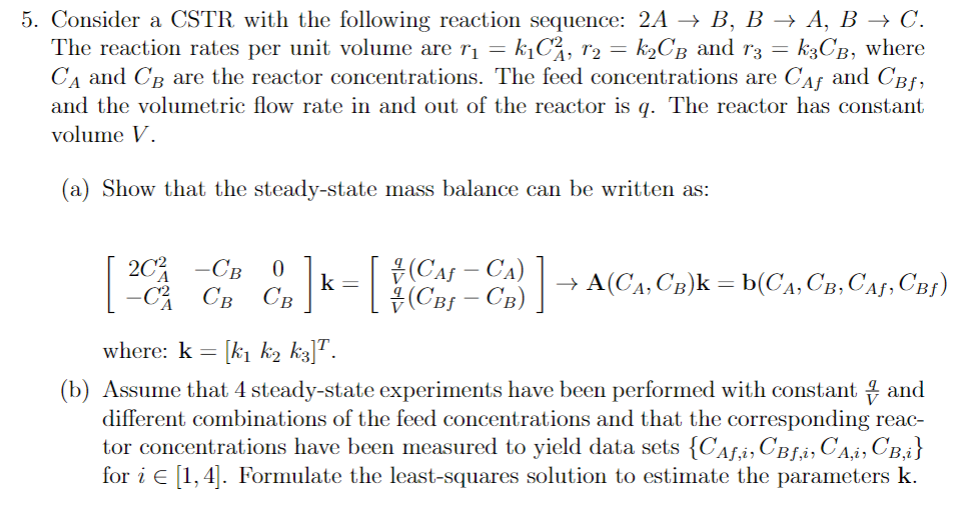
Consider a CSTR with the following reaction sequence: 2 A B , B A , B C . The reaction rates per unit volume are
Consider a CSTR with the following reaction sequence: The reaction rates per unit volume are and where and are the reactor concentrations. The feed concentrations are and and the volumetric flow rate in and out of the reactor is The reactor has constant volume
a Show that the steadystate mass balance can be written as:
where:
b Assume that steadystate experiments have been performed with constant and different combinations of the feed concentrations and that the corresponding reactor concentrations have been measured to yield data sets for Formulate the leastsquares solution to estimate the parameters
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


