Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a cylinder containing an ideal gas under a piston, as shown in Figure below. After a weight m is removed from the piston,
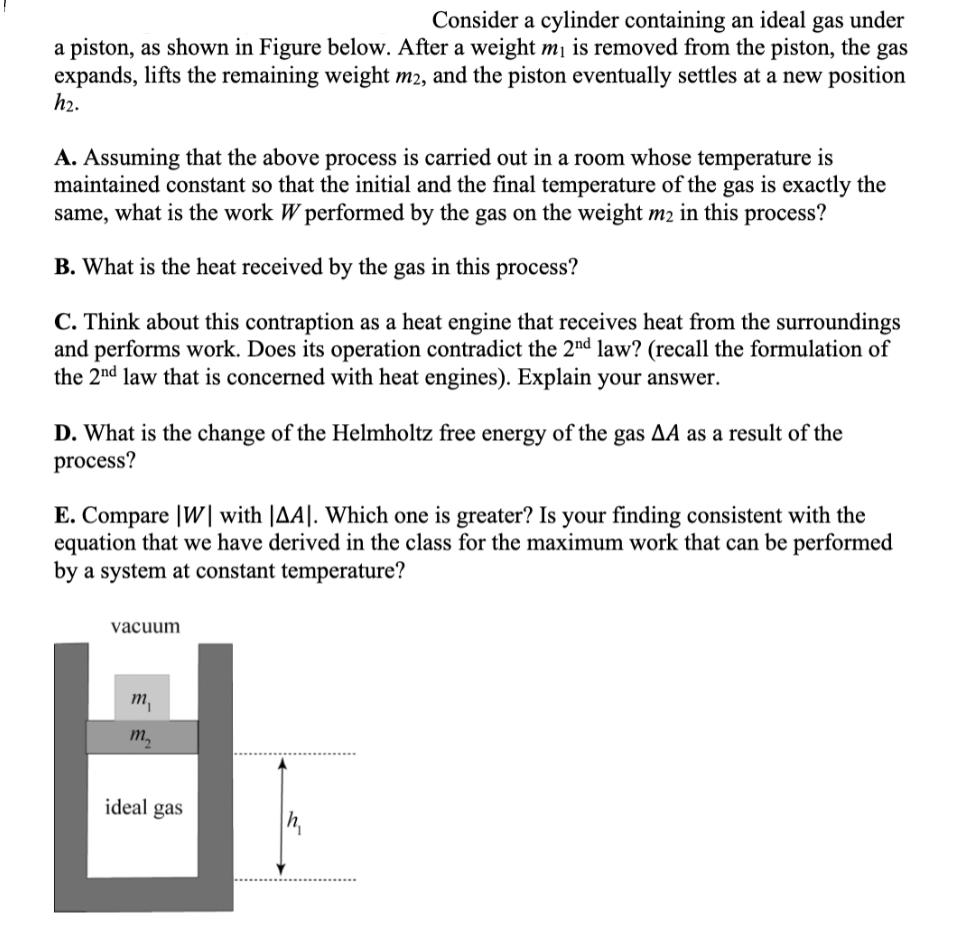
Consider a cylinder containing an ideal gas under a piston, as shown in Figure below. After a weight m is removed from the piston, the gas expands, lifts the remaining weight m2, and the piston eventually settles at a new position h. A. Assuming that the above process is carried out in a room whose temperature is maintained constant so that the initial and the final temperature of the gas is exactly the same, what is the work W performed by the gas on the weight m2 in this process? B. What is the heat received by the gas in this process? C. Think about this contraption as a heat engine that receives heat from the surroundings and performs work. Does its operation contradict the 2nd law? (recall the formulation of the 2nd law that is concerned with heat engines). Explain your answer. D. What is the change of the Helmholtz free energy of the gas AA as a result of the process? E. Compare [W] with |AA|. Which one is greater? Is your finding consistent with the equation that we have derived in the class for the maximum work that can be performed by a system at constant temperature? vacuum m m ideal gas
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


