Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a gas mixture of acetone (1) and methanol (2) with y = 0.514 and liquid mixture of the same components with x =
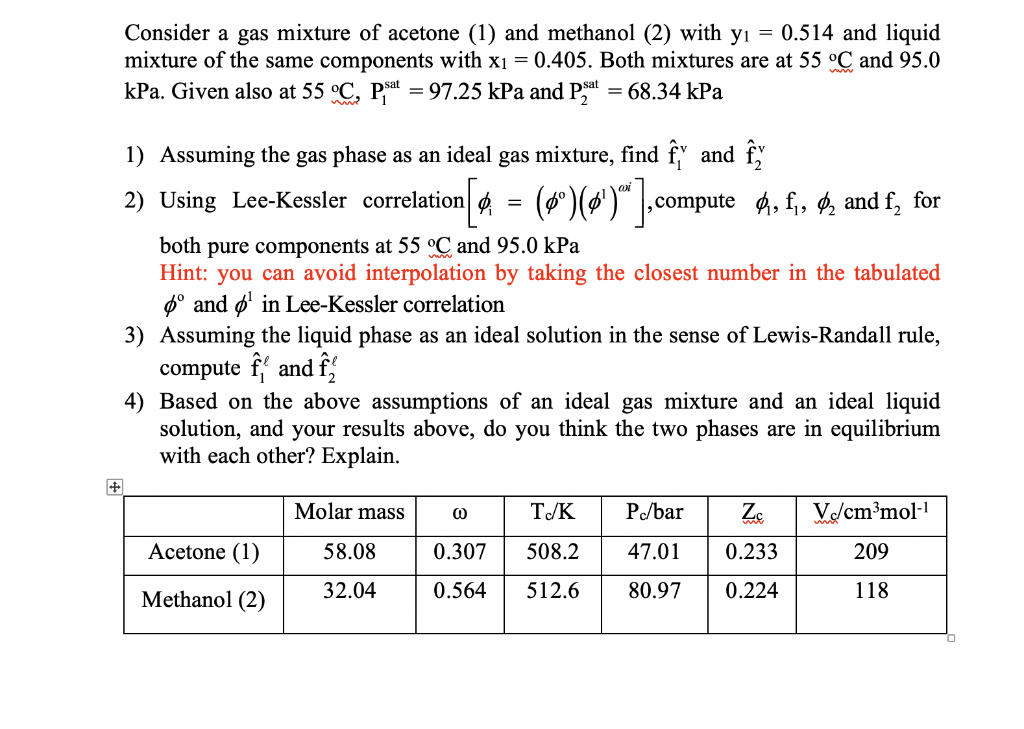
Consider a gas mixture of acetone (1) and methanol (2) with y = 0.514 and liquid mixture of the same components with x = 0.405. Both mixtures are at 55 C and 95.0 kPa. Given also at 55 C, Pat = 97.25 kPa and Pat = 68.34 kPa 1) Assuming the gas phase as an ideal gas mixture, find y and fy 2) Using Lee-Kessler correlation[ = (0) ()" ], compute , f, and f, for both pure components at 55 C and 95.0 kPa Hint: you can avoid interpolation by taking the closest number in the tabulated and in Lee-Kessler correlation 3) Assuming the liquid phase as an ideal solution in the sense of Lewis-Randall rule, compute and f 4) Based on the above assumptions of an ideal gas mixture and an ideal liquid solution, and your results above, do you think the two phases are in equilibrium with each other? Explain. Acetone (1) Methanol (2) Molar mass 58.08 32.04 @ 0.307 0.564 Tc/K P./bar 508.2 512.6 Zc Vc/cmmol-1 209 118 47.01 0.233 80.97 0.224
Step by Step Solution
★★★★★
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


