Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a heterogeneous gas-phase catalytic reaction: Catalyst AB (g) A(g) + B (g) The surface reaction mechanism follows three steps: AB (g) + SAB
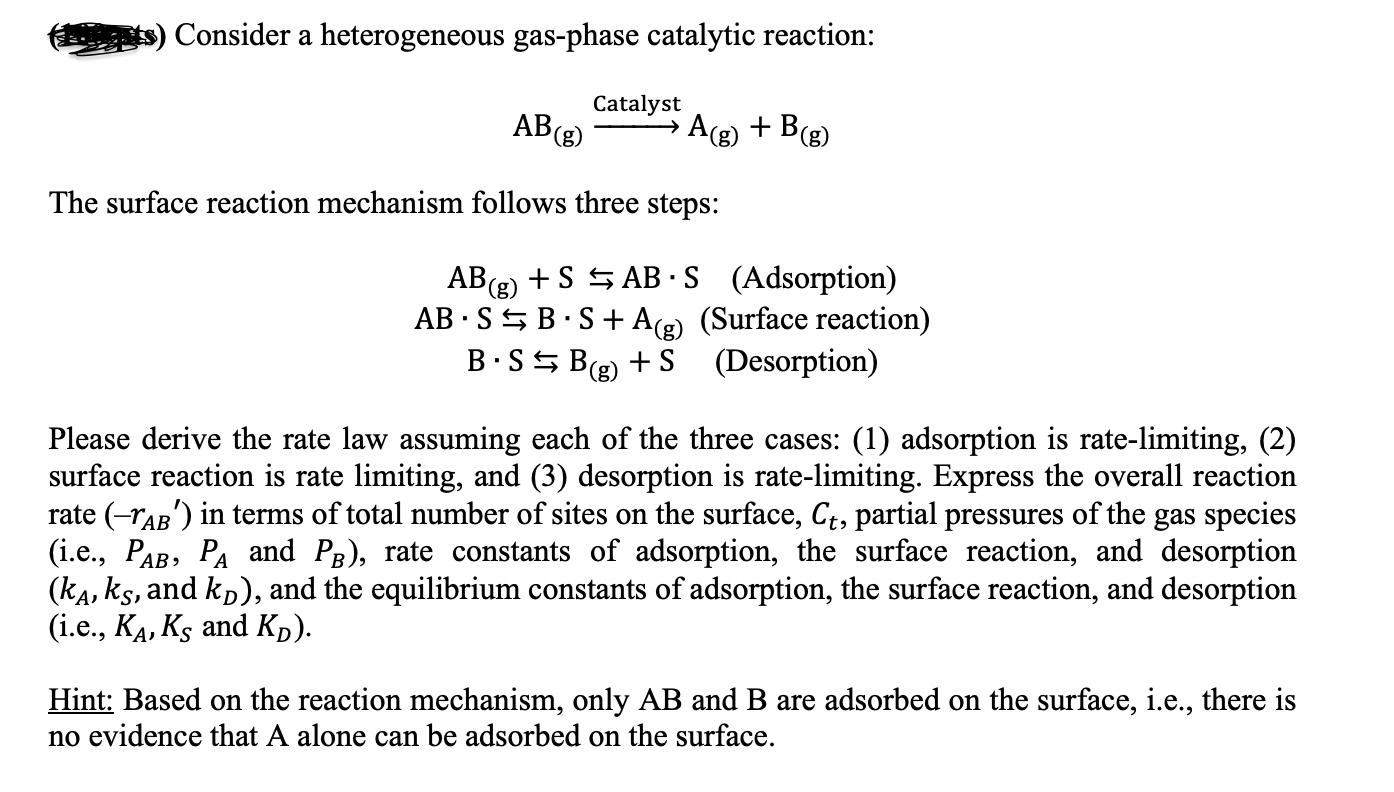
Consider a heterogeneous gas-phase catalytic reaction: Catalyst AB (g) A(g) + B (g) The surface reaction mechanism follows three steps: AB (g) + SAB S (Adsorption) AB SB S+A (g) (Surface reaction) (Desorption) B.SB (g) + S Please derive the rate law assuming each of the three cases: (1) adsorption is rate-limiting, (2) surface reaction is rate limiting, and (3) desorption is rate-limiting. Express the overall reaction rate (AB) in terms of total number of sites on the surface, Ct, partial pressures of the gas species (i.e., PAB, PA and PB), rate constants of adsorption, the surface reaction, and desorption (k, ks, and kn), and the equilibrium constants of adsorption, the surface reaction, and desorption (i.e., KA, KS and KD). Hint: Based on the reaction mechanism, only AB and B are adsorbed on the surface, i.e., there is no evidence that A alone can be adsorbed on the surface.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


