Question
2.5, Consider a mixture of 25% ammonia, the rest nitrogen and hydrogen in a 1:3 ratio. The gas is at 270 atm and 550
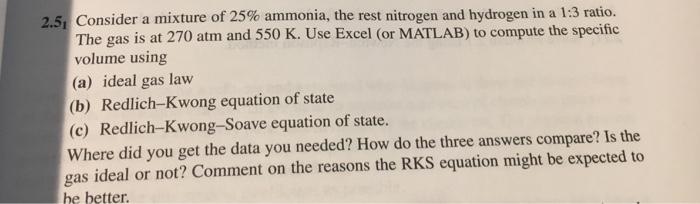
2.5, Consider a mixture of 25% ammonia, the rest nitrogen and hydrogen in a 1:3 ratio. The gas is at 270 atm and 550 K. Use Excel (or MATLAB) to compute the specific volume using (a) ideal gas law (b) Redlich-Kwong equation of state (c) Redlich-Kwong-Soave equation of state. Where did you get the data you needed? How do the three answers compare? Is the gas ideal or not? Comment on the reasons the RKS equation might be expected to he better.
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



