Consider a non-ideal gas that follows the Van der Waals equation of state: RT a v-b 22 P = Where p is the pressure,
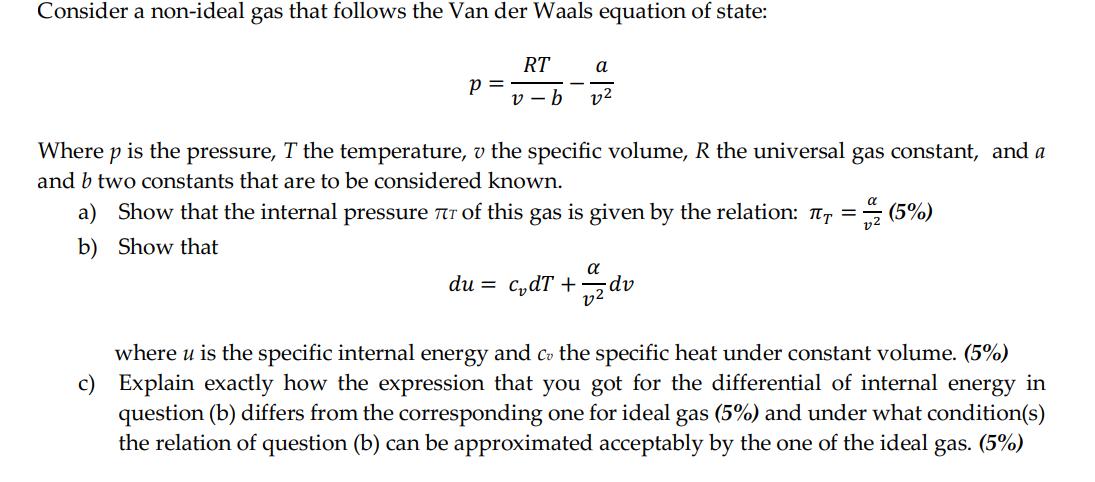
Consider a non-ideal gas that follows the Van der Waals equation of state: RT a v-b 22 P = Where p is the pressure, T the temperature, v the specific volume, R the universal gas constant, and a and b two constants that are to be considered known. a) Show that the internal pressure 7 of this gas is given by the relation: = (5%) b) Show that du = cdT + 2 dv where u is the specific internal energy and co the specific heat under constant volume. (5%) c) Explain exactly how the expression that you got for the differential of internal energy in question (b) differs from the corresponding one for ideal gas (5%) and under what condition(s) the relation of question (b) can be approximated acceptably by the one of the ideal gas. (5%)
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


