Answered step by step
Verified Expert Solution
Question
1 Approved Answer
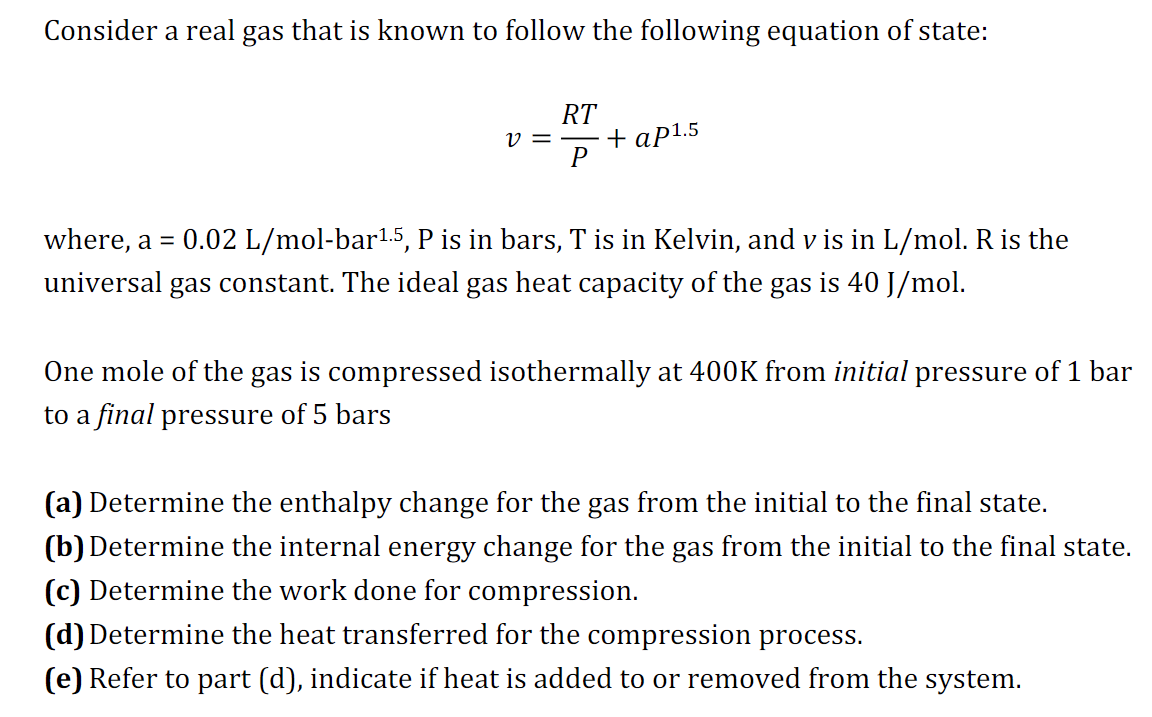
Consider a real gas that is known to follow the following equation of state: v = R T P + a P 1 . 5
Consider a real gas that is known to follow the following equation of state:
where, bar is in bars, is in Kelvin, and is in is the
universal gas constant. The ideal gas heat capacity of the gas is
One mole of the gas is compressed isothermally at from initial pressure of bar
to a final pressure of bars
a Determine the enthalpy change for the gas from the initial to the final state.
b Determine the internal energy change for the gas from the initial to the final state.
c Determine the work done for compression.
d Determine the heat transferred for the compression process.
e Refer to part d indicate if heat is added to or removed from the system.
Please solve it clearly with steps.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


