Question
Consider a stoichiometric mixture of H and O at standard condition (298.15 K and 1.05 x 105 Pa,) at the upstream. The specific heat
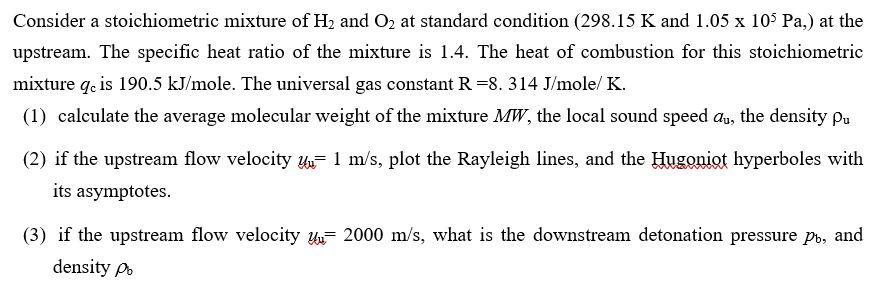
Consider a stoichiometric mixture of H and O at standard condition (298.15 K and 1.05 x 105 Pa,) at the upstream. The specific heat ratio of the mixture is 1.4. The heat of combustion for this stoichiometric mixture qe is 190.5 kJ/mole. The universal gas constant R =8. 314 J/mole/ K. (1) calculate the average molecular weight of the mixture MW, the local sound speed au, the density pu (2) if the upstream flow velocity u 1 m/s, plot the Rayleigh lines, and the Hugoniot hyperboles with its asymptotes. (3) if the upstream flow velocity 2000 m/s, what is the downstream detonation pressure po, and density po
Step by Step Solution
3.59 Rating (199 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTIONS Or 1 calculate the average molecular weight of the mixture MW the local soun...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



