Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a system composed of an ideal gas. (a) (2 points) Derive P(V), this is, pressure as a function of volume, for a reversible
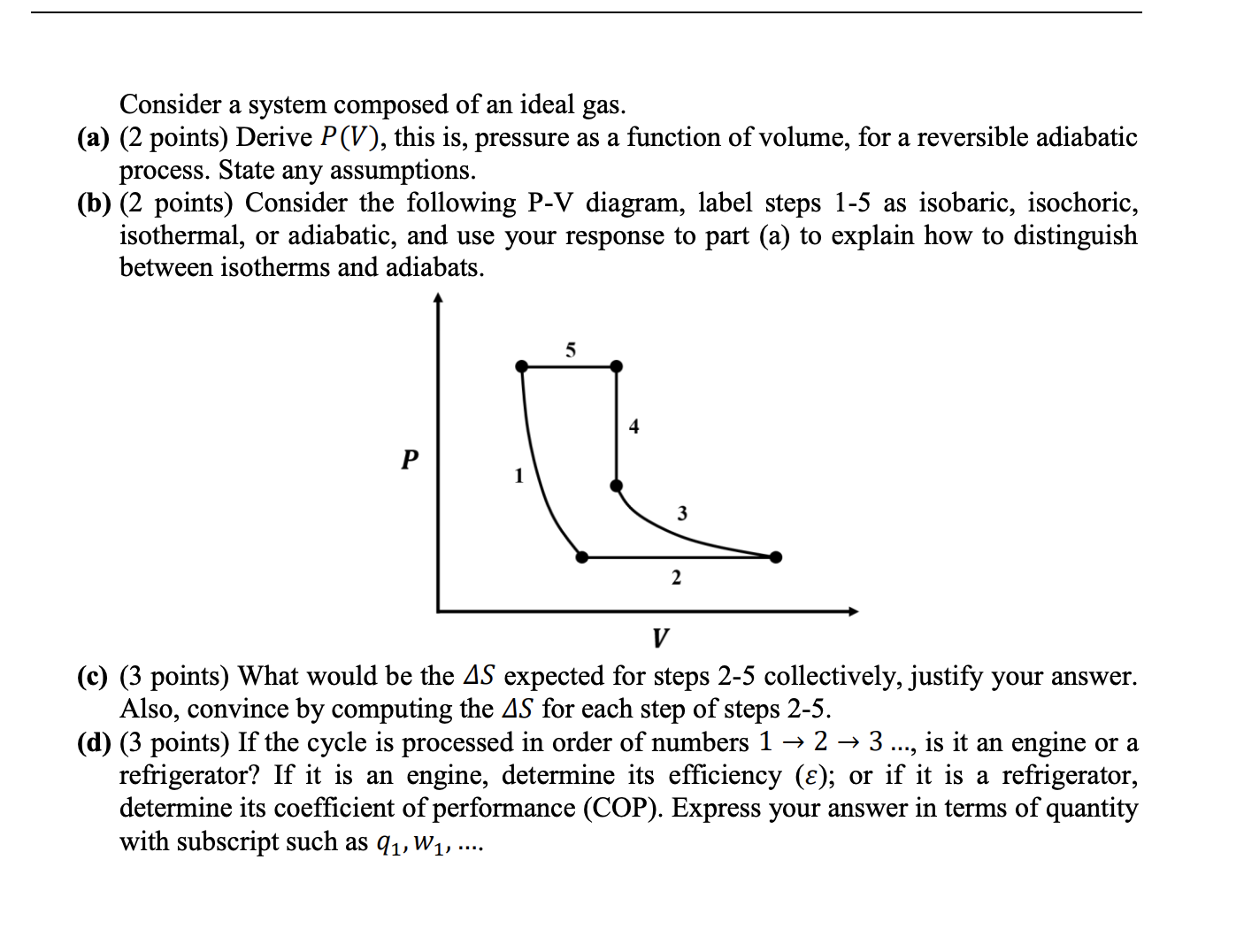
Consider a system composed of an ideal gas. (a) (2 points) Derive P(V), this is, pressure as a function of volume, for a reversible adiabatic process. State any assumptions. (b) (2 points) Consider the following P-V diagram, label steps 1-5 as isobaric, isochoric, isothermal, or adiabatic, and use your response to part (a) to explain how to distinguish between isotherms and adiabats. P 5 4 2 3 V (c) (3 points) What would be the AS expected for steps 2-5 collectively, justify your answer. Also, convince by computing the AS for each step of steps 2-5. (d) (3 points) If the cycle is processed in order of numbers 1 2 3 ..., is it an engine or a refrigerator? If it is an engine, determine its efficiency (); or if it is a refrigerator, determine its coefficient of performance (COP). Express your answer in terms of quantity with subscript such as 91, W1, ...
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


