Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a system consisting of an ideal gas confined within a container, one wall of which is a movable piston. Energy can be added
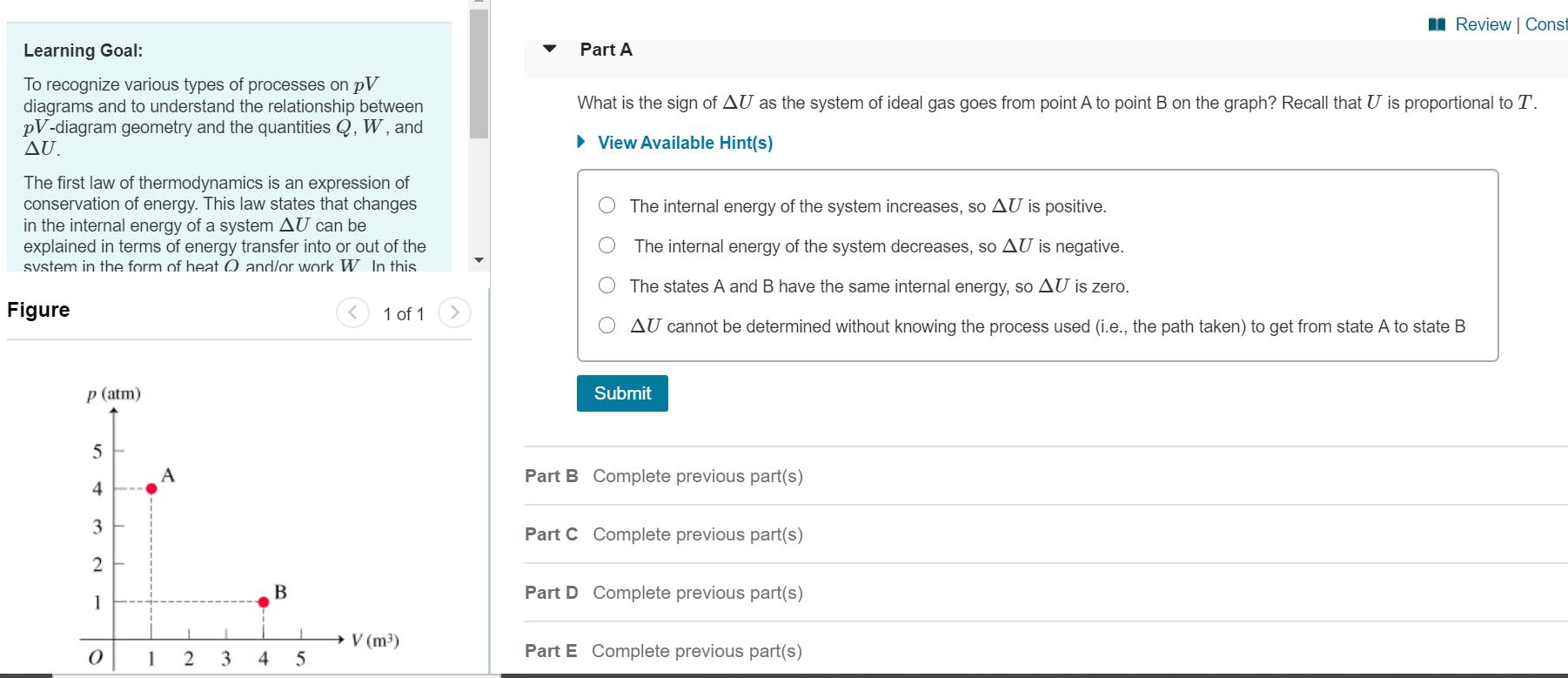
Consider a system consisting of an ideal gas confined within a container, one wall of which is a movable piston. Energy can be added to the gas in the form of heat by applying a flame to the outside of the container. Conversely, energy can also be removed from the gas in the form of heat by immersing the container in ice water. Energy can be added to the system in the form of work by pushing the piston in, thereby compressing the gas. Conversely, if the gas pushes the piston out, thereby pushing some atmosphere aside, the internal energy of the gas is reduced by the amount of work done. The internal energy of an ideal gas is directly proportional to its absolute temperature T. An ideal gas also obeys the ideal gas law pV = nRT, so the absolute temperature T is directly proportional to the product of the absolute pressure p and the volume V. Heren denotes the amount of gas in moles, which is a constant because the gas is confined, and R is the universal gas constant. ApV diagram (Figure 1) is a convenient way to track the pressure and volume of a system. Energy transfers by heat and/or work are associated with processes, which are lines or curves on the pV diagram taking the system from one state (i.e., one point on the diagram) to another. Work corresponds geometrically to the area under the curve on a pV diagram. If the volume increases (i.e., the system expands) the work will be classified as an energy output from the system. I Review | Const Learning Goal: Part A To recognize various types of processes on pV diagrams and to understand the relationship between pV-diagram geometry and the quantities Q, W, and AU. What is the sign of AU as the system of ideal gas goes from point A to point B on the graph? Recall that U is proportional to T. View Available Hint(s) The first law of thermodynamics is an expression conservation of energy. This law states that changes in the internal energy of a system AU can be explained in terms of energy transfer into or out of the svstem in the form of heat 0 and/or work W. In this The internal energy of the system increases, so AU is positive. The internal energy of the system decreases, so AU is negative. The states A and B have the same internal energy, so AU is zero. Figure 1 of 1 AU cannot be determined without knowing the process used (i.e., the path taken) to get from state A to state B p (atm) Submit 5 A Part B Complete previous part(s) 4 3 Part C Complete previous part(s) Part D Complete previous part(s) 1 V (m3) 1 3 4 Part E Complete previous part(s)
Step by Step Solution
★★★★★
3.34 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
option ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635e055d517e2_180795.pdf
180 KBs PDF File
635e055d517e2_180795.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


