Answered step by step
Verified Expert Solution
Question
1 Approved Answer
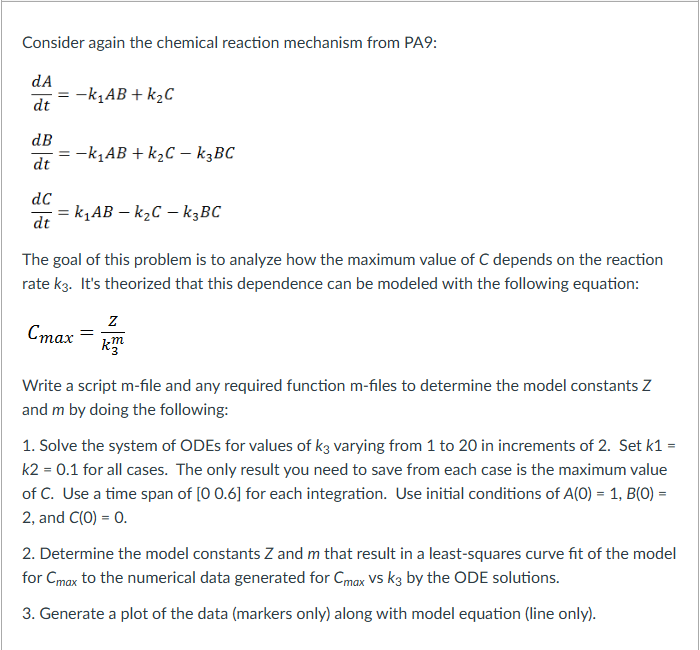
Consider again the chemical reaction mechanism from PA 9 : d A d t = - k 1 A B + k 2 C d
Consider again the chemical reaction mechanism from PA:
The goal of this problem is to analyze how the maximum value of depends on the reaction
rate It's theorized that this dependence can be modeled with the following equation:
Write a script file and any required function files to determine the model constants
and by doing the following:
Solve the system of ODEs for values of varying from to in increments of Set
for all cases. The only result you need to save from each case is the maximum value
and
Determine the model constants and that result in a leastsquares curve fit of the model
for to the numerical data generated for vs by the ODE solutions.
Generate a plot of the data markers only along with model equation line only
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


