Question
Consider an anionic polymerization of 1.0 mol of styrene in tetrahydrofuran solution with 1.0x10- mol of n-BuLi initiator. At 50% conversion, 0.25x10- mol of
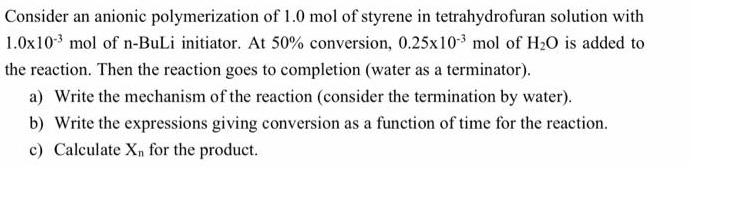
Consider an anionic polymerization of 1.0 mol of styrene in tetrahydrofuran solution with 1.0x10- mol of n-BuLi initiator. At 50% conversion, 0.25x10- mol of HO is added to the reaction. Then the reaction goes to completion (water as a terminator). a) Write the mechanism of the reaction (consider the termination by water). b) Write the expressions giving conversion as a function of time for the reaction. c) Calculate Xn for the product.
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
a The mechanism of the reaction is as follows nBuLi initiates the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



