Question
Consider an equilibrium C-O-S gas phase, containing CO, CO, COS, and S0 2 T = 1000 K PCO = 0.002 atm PCO2=0.30 atm PSO2=0.01
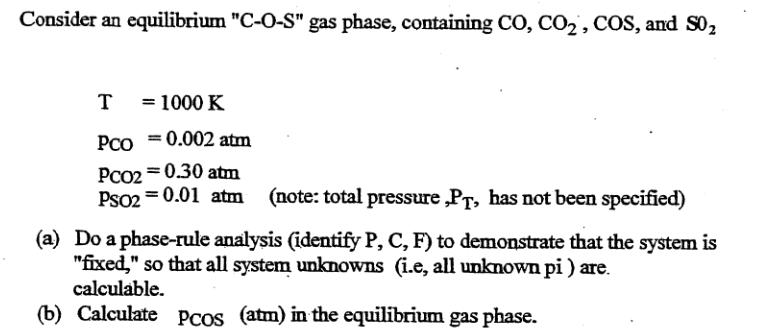

Consider an equilibrium "C-O-S" gas phase, containing CO, CO, COS, and S0 2 T = 1000 K PCO = 0.002 atm PCO2=0.30 atm PSO2=0.01 atm (note: total pressure,PT, has not been specified) (a) Do a phase-rule analysis (identify P, C, F) to demonstrate that the system is "fixed," so that all system unknowns (i.e, all unknown pi ) are. calculable. (b) Calculate Pcos (atm) in the equilibrium gas phase. Species O(g) $(g) C(s) CO(g) CO(g) COS(g) SO(g) Mn(s) MnO(s) 2.0x1016 Mn203(s) 1.9x1035 Mn3O4(s) 1.2x1054 MnS(s) K1000 1 1 1 3.0x1010 4.8x1020 2.2x1011 1.2*1015 1 4.6x1010 Kf are for the reactions forming one mole of the compound from the elements: O(g), S2(g), C(s) with all reactants and products present in their standard states.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



