Question
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm. Calculate the work, w, if the gas expands
Consider an ideal gas enclosed in a 1.00 L container at an internal pressure of 10.0 atm.
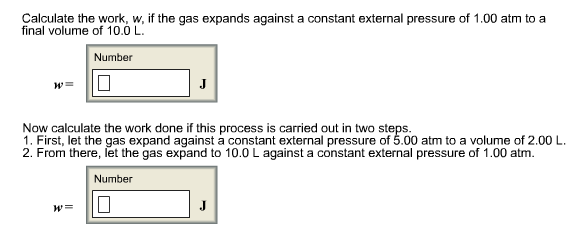
Calculate the work, w, if the gas expands against a constant external pressure of 1.00 atm to a final volume of 10.0 L. Number W= J Now calculate the work done if this process is carried out in two steps. 1. First, let the gas expand against a constant external pressure of 5.00 atm to a volume of 2.00 L. 2. From there, let the gas expand to 10.0 L against a constant external pressure of 1.00 atm. Number 0 W= J
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
The problem is based on the concept of thermodynamics Thermodynamics deals with the change with ener...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



