Question
Consider an incident electron with 14.2 eV of energy. It collides with an electron of an unexcited gas atom (described by the following energy
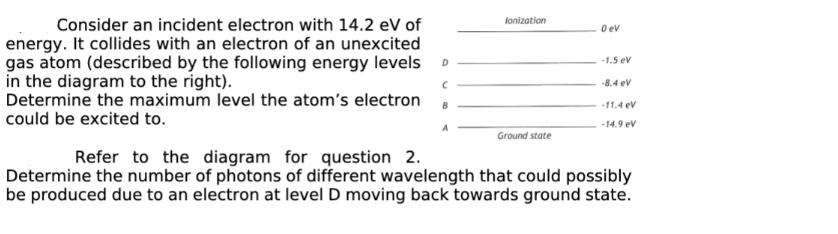
Consider an incident electron with 14.2 eV of energy. It collides with an electron of an unexcited gas atom (described by the following energy levels D in the diagram to the right). C Determine the maximum level the atom's electron B could be excited to. lonization Ground state 0 ev -1.5 eV -8.4 eV -11.4 eV -14.9 ev Refer to the diagram for question 2. Determine the number of photons of different wavelength that could possibly be produced due to an electron at level D moving back towards ground state.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The image you provided contains a diagram alongside a twopart question related to the energy levels ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
University Physics with Modern Physics
Authors: Hugh D. Young, Roger A. Freedman
14th edition
133969290, 321973615, 9780321973610, 978-0133977981
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



