Question: Consider an ionic compound, MX,, composed of generic metal M and generic gaseous halogen X. The enthalpy of formation of MX, is AH; =

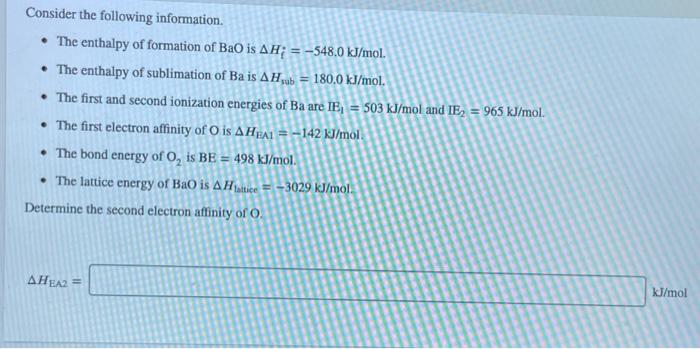
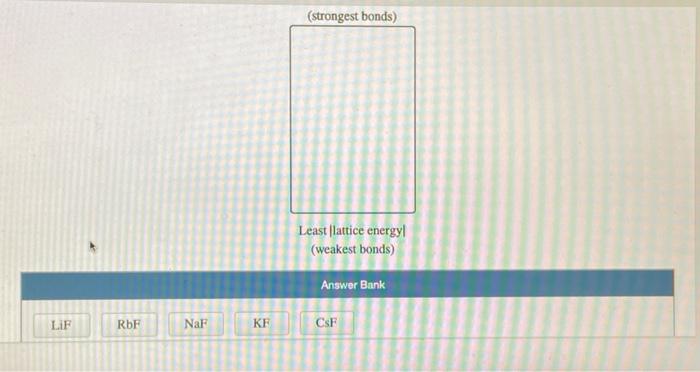
Consider an ionic compound, MX,, composed of generic metal M and generic gaseous halogen X. The enthalpy of formation of MX, is AH; = -797 kJ/mol. The enthalpy of sublimation of M is A Hub = 171 kJ/mol. The first, second, and third ionization energies of M are IE, = 565 kJ/mol, IE = 1665 kJ/mol, and IE3 = 2527 kJ/mol. The electron affinity of X is AHEA = -373 kJ/mol. (Refer to the hint). The bond energy of X, is BE = 185 kJ/mol. Determine the lattice energy of MX,. AH lattice = kJ/mol Consider the following information. The enthalpy of formation of BaO is AH; = -548.0 kJ/mol. The enthalpy of sublimation of Ba is AHub = 180.0 kJ/mol. The first and second ionization energies of Ba are IE = 503 kJ/mol and IE = 965 kJ/mol. The first electron affinity of O is AHEA1-142 kJ/mol. The bond energy of O, is BE = 498 kJ/mol. The lattice energy of BaO is AHattice-3029 kJ/mol Determine the second electron affinity of O. AHEA2 = kJ/mol LIF RbF NaF KF (strongest bonds) Least lattice energyl (weakest bonds) Answer Bank CSF
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


