Question
Consider an ionic crystal consisting of a pair of oppositely charged ions. The cation specie has a valence of +1, a radius of 0.11
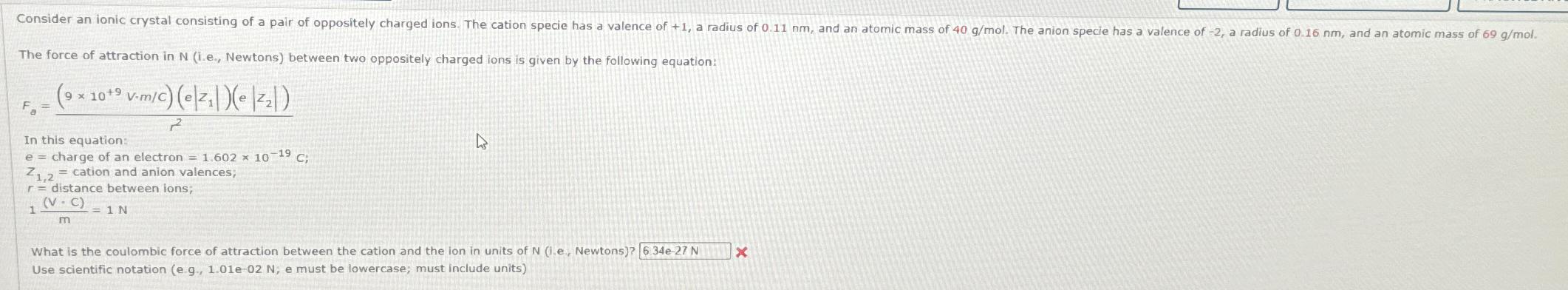
Consider an ionic crystal consisting of a pair of oppositely charged ions. The cation specie has a valence of +1, a radius of 0.11 nm, and an atomic mass of 40 g/mol. The anion specie has a valence of -2, a radius of 0.16 nm, and an atomic mass of 69 g/mol. The force of attraction in N (i.e., Newtons) between two oppositely charged ions is given by the following equation: (9 x 10+9 v-m/c) (e|z|)(e||) 2 F = In this equation: e = charge of an electron = 1.602 10-19 C; Z1,2 = cation and anion valences; r = distance between ions; (V-C) 1 = 1 N m D What is the coulombic force of attraction between the cation and the ion in units of N (1.e., Newtons)? 6.34e-27 N Use scientific notation (e g., 1.01e-02 N, e must be lowercase; must include units) X
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Shigleys Mechanical Engineering Design
Authors: Richard G. Budynas, J. Keith Nisbett
9th edition
77679520, 73529281, 1259986241, 978-0077679521, 9780073529288, 9781259986246, 978-1121345317
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



