Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider an isothermal CSTR with volume = 1, feed concentration Caf= 1, and feed volumetric flow rate vo = 1 (arbitrary volume, concentration, and
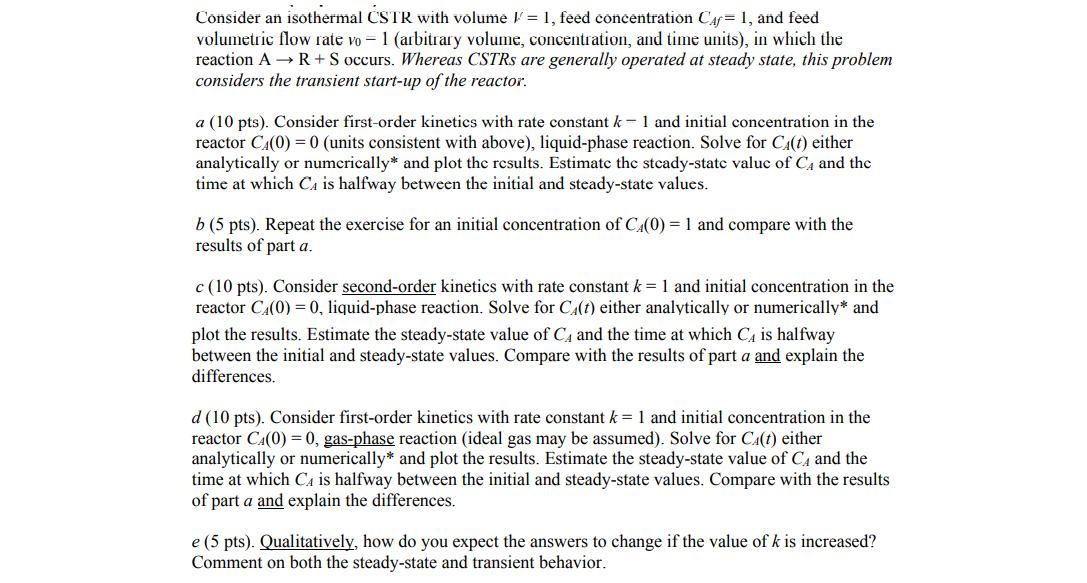
Consider an isothermal CSTR with volume = 1, feed concentration Caf= 1, and feed volumetric flow rate vo = 1 (arbitrary volume, concentration, and time units), in which the reaction A R + S occurs. Whereas CSTRS are generally operated at steady state, this problem considers the transient start-up of the reactor. a (10 pts). Consider first-order kinetics with rate constant k- 1 and initial concentration in the reactor C4(0) =0 (units consistent with above), liquid-phase reaction. Solve for C(t) either analytically or numcrically* and plot the results. Estimate the stcady-state valuc of C, and the time at which Ca is halfway between the initial and steady-state values. b (5 pts). Repeat the exercise for an initial concentration of C4(0) = 1 and compare with the results of part a. c (10 pts). Consider second-order kinetics with rate constant k= 1 and initial concentration in the reactor C4(0) = 0, liquid-phase reaction. Solve for C4(t) either analytically or numerically* and plot the results. Estimate the steady-state value of C4 and the time at which C4 is halfway between the initial and steady-state values. Compare with the results of part a and explain the differences. d (10 pts). Consider first-order kinetics with rate constant k = 1 and initial concentration in the reactor CA(0) = 0, gas-phase reaction (ideal gas may be assumed). Solve for CA(t) either analytically or numerically* and plot the results. Estimate the steady-state value of C4 and the time at which CA is halfway between the initial and steady-state values. Compare with the results of part a and explain the differences. e (5 pts). Qualitatively, how do you expect the answers to change if the value of k is increased? Comment on both the steady-state and transient behavior.
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Answer Solution Fay CSTR FA Mole balamce 4 A ta C...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


