Question
Consider the ammonia process given below. A feed of 20% N2, 78% H2 and 2% CH4 is mixed with two recycle streams and enters a
Consider the ammonia process given below. A feed of 20% N2, 78% H2 and 2% CH4 is mixed with two recycle streams and enters a reactor. Here, conversion per pass of N2 to NH3 is 45% according to the reaction:
N2 + 3H2 --> 2NH3
The ammonia product is recovered by flashing the reactor effluent in two stages and recycling the overhead vapour. If the purge fraction is 5% for the high pressure recycle, what is the methane concentration in the reactor feed?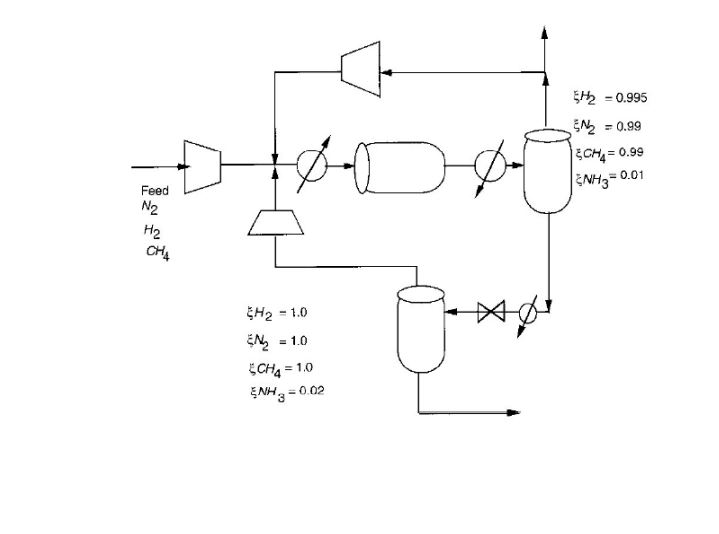
Feed N H CHA 2 H = 1.0 EN = 1.0 CH4 = 1.0 NH3 = 0.02 H = 0.995 = 0.99 CH=0.99 NH3=0.01
Step by Step Solution
There are 3 Steps involved in it
Step: 1
SOLUTION according to mass balance the mass of th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



