Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the candle shown in the figure below. After the candle is lit the wax next to the flame soon reaches its melting temperature (
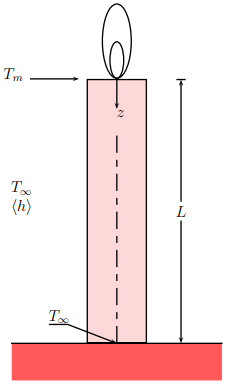
Consider the candle shown in the figure below. After the candle is lit the wax next to the flame soon reaches its melting temperature Tm and the candle begins to melt. Assume that after certain time the candle starts to glow steadily. Let the length of the candle be L and the radius be R The temperature at the base of the candle is Tinf and the temperature of the surrounding air is also Tinf The candle losses some heat to the surrounding by convection along the length of the candle. The average convective heat transfer coefficient is h For simplicity, assume that the melting occurs slowly enough that pseudo steady state approximation can be used.
a Find the temperature profile Tz in the candle, assuming that the temperature is approximately independent of radial position.
b Calculate the amount of heat Q enters the candle at z
c Assuming that the intial length of the candle is L and the heat loss by radiation and convection at z is negligible, what is the time required for the candle to melt completely?
HINT: Assume that the candle as our system, write an unsteady state energy balance. An unsteadystate balance on the candle gives: Accumulation rate in The rate heat into the candle is derived in part b The latend heat of melting of wax is lambdasl
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


