Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the energy levels of a hydrogenic atom for which $n$=3. In terms of appropriate quantum number values, list the different eigenstates, first assuming no
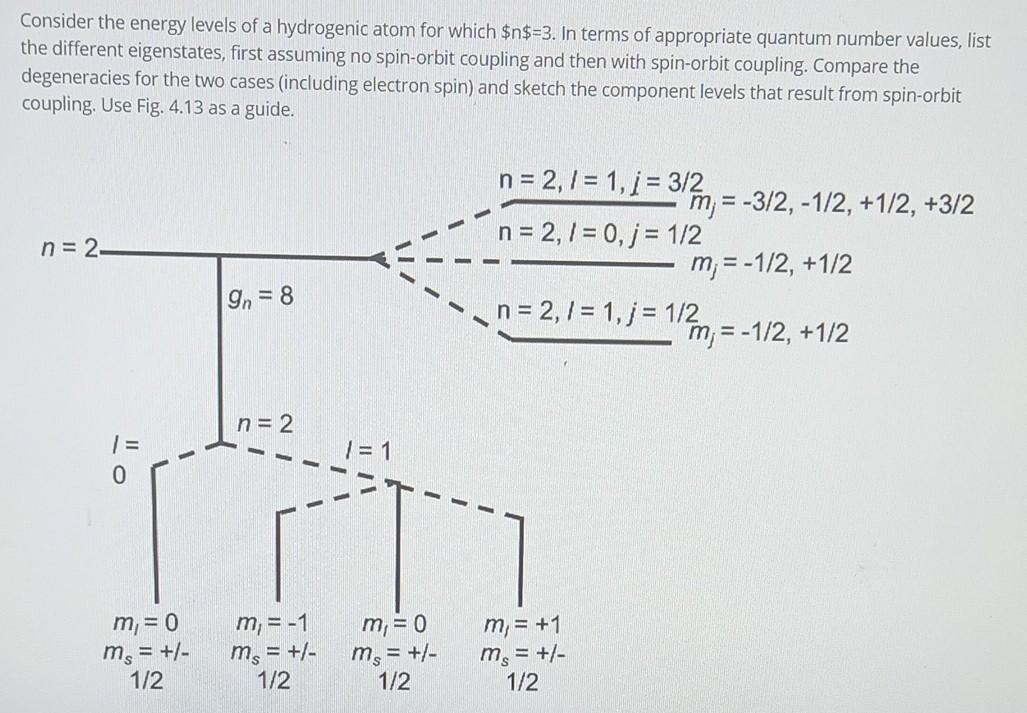
Consider the energy levels of a hydrogenic atom for which $n$=3. In terms of appropriate quantum number values, list the different eigenstates, first assuming no spin-orbit coupling and then with spin-orbit coupling. Compare the degeneracies for the two cases (including electron spin) and sketch the component levels that result from spin-orbit coupling. Use Fig. 4.13 as a guide. - n = 2 n = 2,7 = 1, 1 = 3/2 m = -3/2, -1/2, +1/2, +3/2 n=2,1 = 0, j = 1/2 m;= -1/2, +1/2 .. n=2,1 = 1, j = 1/2 m= -1/2, +1/2 On = 8 n= 2 = To IN 1 = 1 - m= 0 ms = +/- 1/2 m, = -1 ms = +/- 1/2 m,= 0 m = +/- 1/2 m = +1 ms = +/- 1/2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


