Question
Consider the following Bronsted-Lowry equilibrium: C6HSNH2(aq) + H2Om C6H&NH3 (ng) + OH (aq) The substances acting as acids and bases from left to right
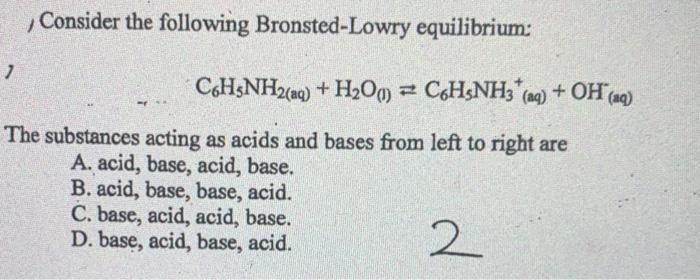
Consider the following Bronsted-Lowry equilibrium: C6HSNH2(aq) + H2Om C6H&NH3 (ng) + OH (aq) The substances acting as acids and bases from left to right are A. acid, base, acid, base. B. acid, base, base, acid. C. base, acid, acid, base. D. base, acid, base, acid. 2
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus
Authors: Dale Varberg, Edwin J. Purcell, Steven E. Rigdon
9th edition
131429248, 978-0131429246
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



