Answered step by step
Verified Expert Solution
Question
1 Approved Answer
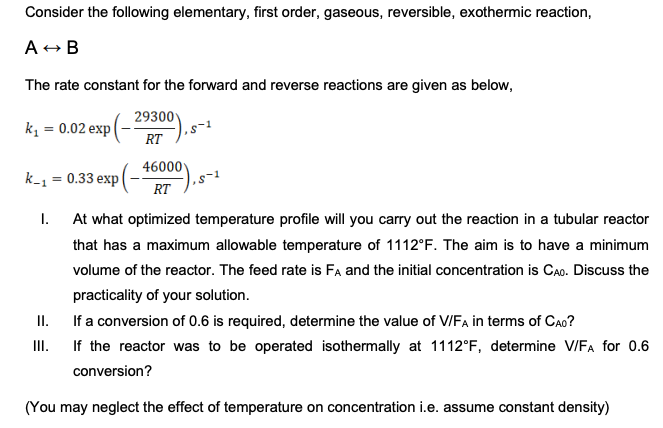
Consider the following elementary, first order, gaseous, reversible, exothermic reaction, A = B The rate constant for the forward and reverse reactions are given as
Consider the following elementary, first order, gaseous, reversible, exothermic reaction,
The rate constant for the forward and reverse reactions are given as below,
exp
exp
I. At what optimized temperature profile will you carry out the reaction in a tubular reactor
that has a maximum allowable temperature of The aim is to have a minimum
volume of the reactor. The feed rate is and the initial concentration is Discuss the
practicality of your solution.
II If a conversion of is required, determine the value of in terms of
III. If the reactor was to be operated isothermally at determine for
conversion?
You may neglect the effect of temperature on concentration ie assume constant density
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


