Answered step by step
Verified Expert Solution
Question
1 Approved Answer
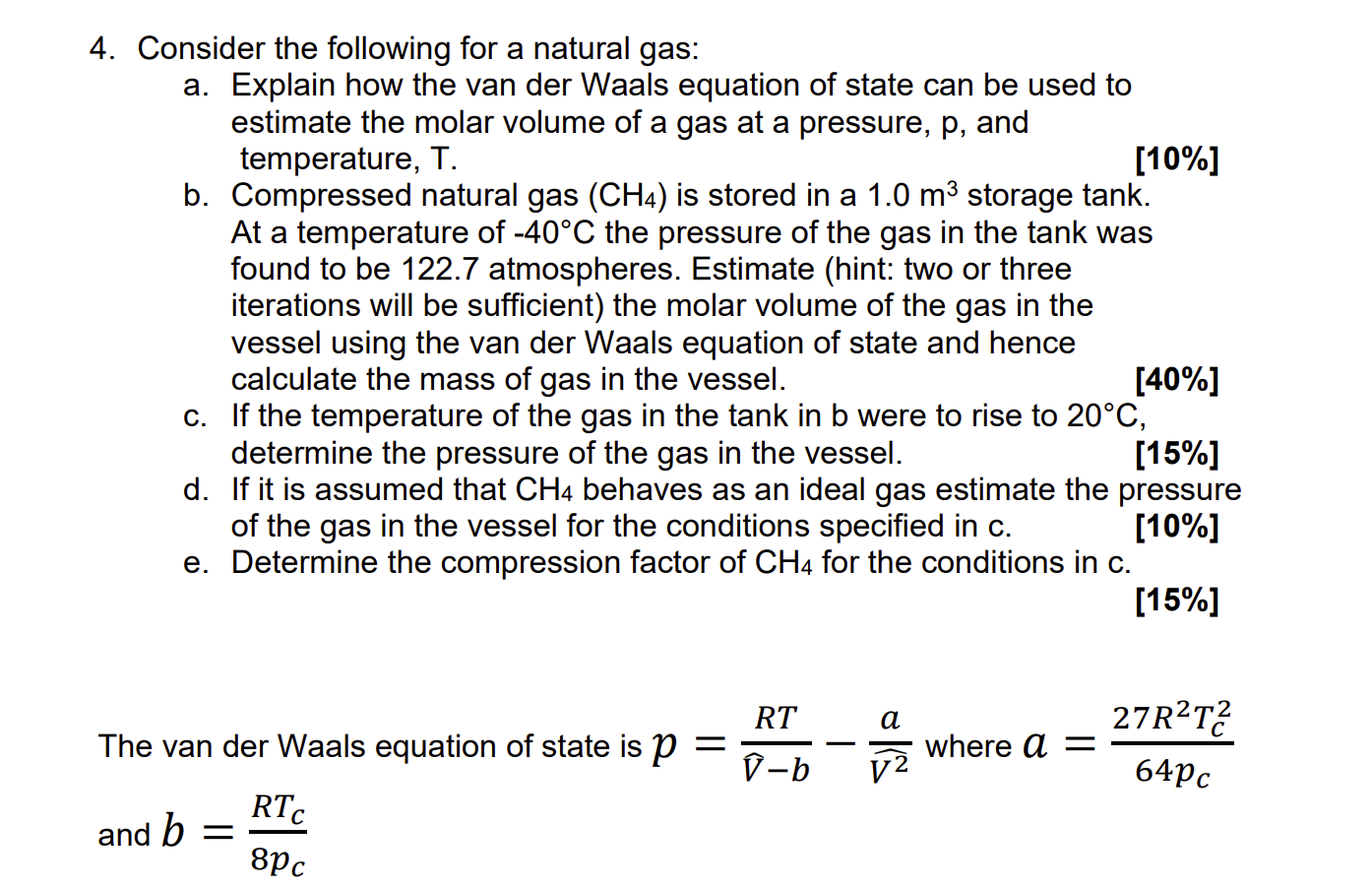
Consider the following for a natural gas: a . Explain how the van der Waals equation of state can be used to estimate the molar
Consider the following for a natural gas:
a Explain how the van der Waals equation of state can be used to
estimate the molar volume of a gas at a pressure, and
temperature,
b Compressed natural gas is stored in a storage tank.
At a temperature of the pressure of the gas in the tank was
found to be atmospheres. Estimate hint: two or three
iterations will be sufficient the molar volume of the gas in the
vessel using the van der Waals equation of state and hence
calculate the mass of gas in the vessel.
c If the temperature of the gas in the tank in b were to rise to
determine the pressure of the gas in the vessel.
d If it is assumed that behaves as an ideal gas estimate the pressure
of the gas in the vessel for the conditions specified in
e Determine the compression factor of for the conditions in
The van der Waals equation of state is where
and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


