Question
Estimate the value of K, at 125C for the reaction: 2 SO, (g) + O2(g) 2 SO, (g). Consider the equilibrium: 2 HI (g)
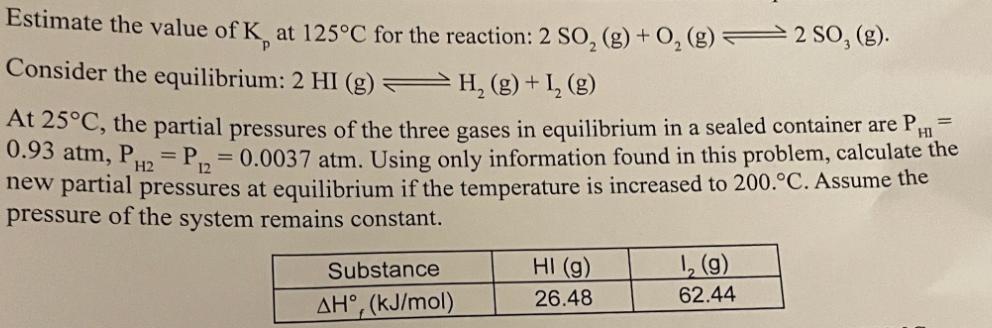
Estimate the value of K, at 125C for the reaction: 2 SO, (g) + O2(g) 2 SO, (g). Consider the equilibrium: 2 HI (g) H (g) + I2 (g) HI At 25C, the partial pressures of the three gases in equilibrium in a sealed container are P, 0.93 atm, P2 P=0.0037 atm. Using only information found in this problem, calculate the new partial pressures at equilibrium if the temperature is increased to 200.C. Assume the pressure of the system remains constant. Substance HI (g) 12(g) AH, (kJ/mol) 26.48 62.44
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
1 Given 2 SO2g O2g 2 SO3g 2 We know that Kp increases as temperature increases for endothermic react...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Accounting
Authors: Jeffrey Waybright, Liang Hsuan Chen, Rhonda Pyper
1st Canadian Edition
9780132147538, 132889714, 013214753X , 978-0132889711
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



