Question
Consider the following pair of reactions. Predict the type of substitution mechanism, predict which reaction of the pair will occur at the faster rate,
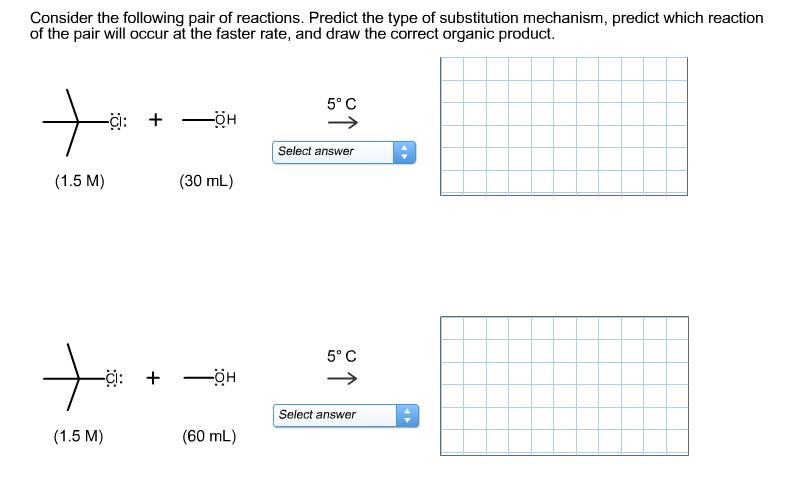
Consider the following pair of reactions. Predict the type of substitution mechanism, predict which reaction of the pair will occur at the faster rate, and draw the correct organic product. + (1.5 M) -C: + + (1.5 M) -ci: -OH (30 mL) + -OH (60 mL) 5 C Select answer 5 C 1 Select answer 41
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Here the reaction proceed through SN path way Mechanism t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Data Mining
Authors: Pang Ning Tan, Michael Steinbach, Vipin Kumar
1st edition
321321367, 978-0321321367
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



