Answered step by step
Verified Expert Solution
Question
1 Approved Answer
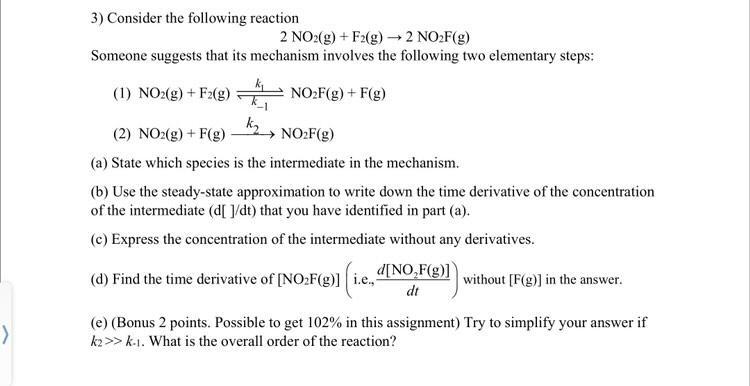
Consider the following reaction 2 N O 2 ( g ) + F 2 ( g ) 2 N O 2 F ( g )
Consider the following reaction
Someone suggests that its mechanism involves the following two elementary steps:
a State which species is the intermediate in the mechanism.
b Use the steadystate approximation to write down the time derivative of the concentration of the intermediate that you have identified in part a
c Express the concentration of the intermediate without any derivatives.
d Find the time derivative of ie: without in the answer.
eBonus points. Possible to get in this assignment Try to simplify your answer if What is the overall order of the reaction?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


