Question
Consider the following reaction: 4 HCI (g) + O2 (g) 2 2C12 (g) + 2H20 (g) Predict in which direction equilibrium moves when the
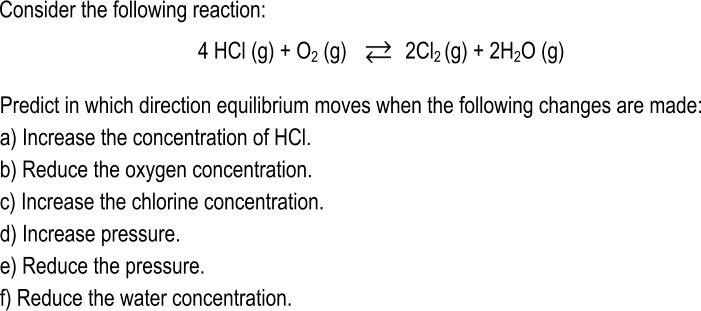
Consider the following reaction: 4 HCI (g) + O2 (g) 2 2C12 (g) + 2H20 (g) Predict in which direction equilibrium moves when the following changes are made: a) Increase the concentration of HCI. b) Reduce the oxygen concentration. c) Increase the chlorine concentration. d) Increase pressure. e) Reduce the pressure. f) Reduce the water concentration.
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
When the equilibrium is disturbed by changing concentration pressure or temperature system will try ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



