Answered step by step
Verified Expert Solution
Question
1 Approved Answer
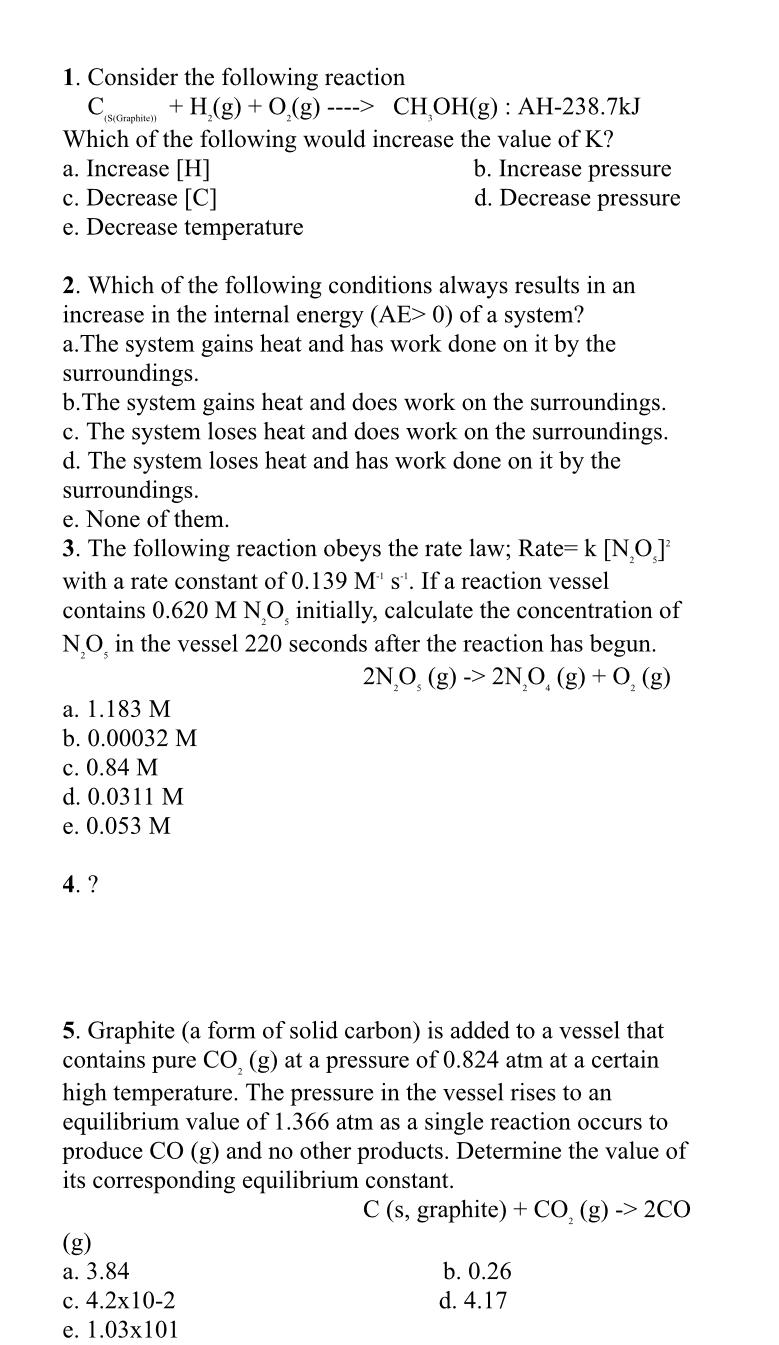
Consider the following reaction Which of the following would increase the value of K ? a . Increase H b . Increase pressure c .
Consider the following reaction
Which of the following would increase the value of
a Increase
b Increase pressure
c Decrease C
d Decrease pressure
e Decrease temperature
Which of the following conditions always results in an increase in the internal energy of a system?
aThe system gains heat and has work done on it by the surroundings.
bThe system gains heat and does work on the surroundings.
c The system loses heat and does work on the surroundings.
d The system loses heat and has work done on it by the surroundings.
e None of them.
The following reaction obeys the rate law; Rate with a rate constant of If a reaction vessel contains initially, calculate the concentration of in the vessel seconds after the reaction has begun.
a
b
c
d
e
Graphite a form of solid carbon is added to a vessel that contains pure at a pressure of atm at a certain high temperature. The pressure in the vessel rises to an equilibrium value of atm as a single reaction occurs to produce CO and no other products. Determine the value of its corresponding equilibrium constant.
graphite
g
a
b
c
d
e
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


