Answered step by step
Verified Expert Solution
Question
1 Approved Answer
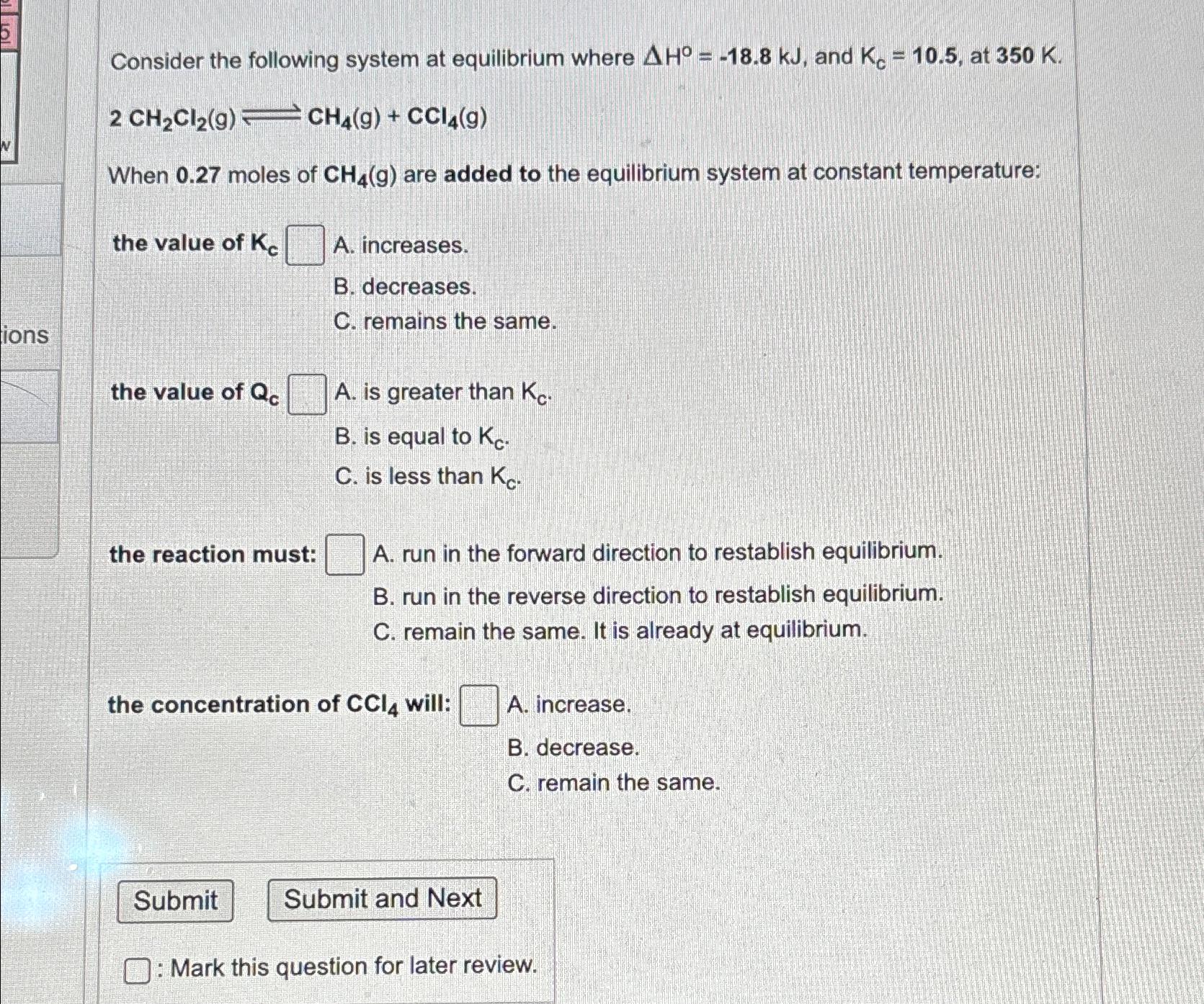
Consider the following system at equilibrium where H = - 1 8 . 8 k J , and K c = 1 0 . 5
Consider the following system at equilibrium where and at
When moles of are added to the equilibrium system at constant temperature:
the value of
A increases.
B decreases.
C remains the same.
the value of
A is greater than
B is equal to
C is less than
the reaction must:
A run in the forward direction to restablish equilibrium.
B run in the reverse direction to restablish equilibrium.
C remain the same. It is already at equilibrium.
the concentration of will:
A increase.
B decrease.
C remain the same.
: Mark this question for later review.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


