Question
Consider the following transformation in which two of the carbon atoms change oxidation state. In order to be an oxidation or reduction, both carbons
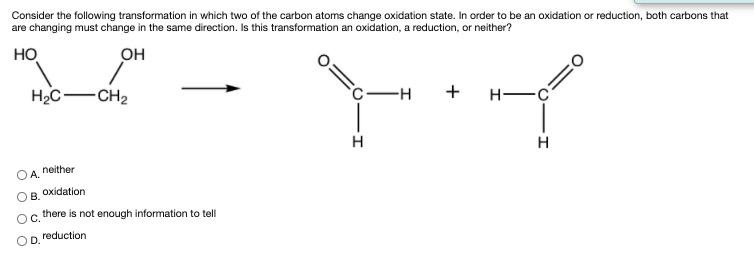
Consider the following transformation in which two of the carbon atoms change oxidation state. In order to be an oxidation or reduction, both carbons that are changing must change in the same direction. Is this transformation an oxidation, a reduction, or neither? H2C- -CH2 c-H H -C H H neither A. oxidation . there is not enough information to tell OC. reduction OD.
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Strategic Compensation A Human Resource Management Approach
Authors: Joseph J. Martocchio
8th edition
978-0133457100, 133457109, 978-0133802023
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



