Question
Consider the freezing of water (or any aqueous solution - including wine). H, O(1) = H,O(s) AH = -6.007 kJ/mol. Calculate the equilibrium constants
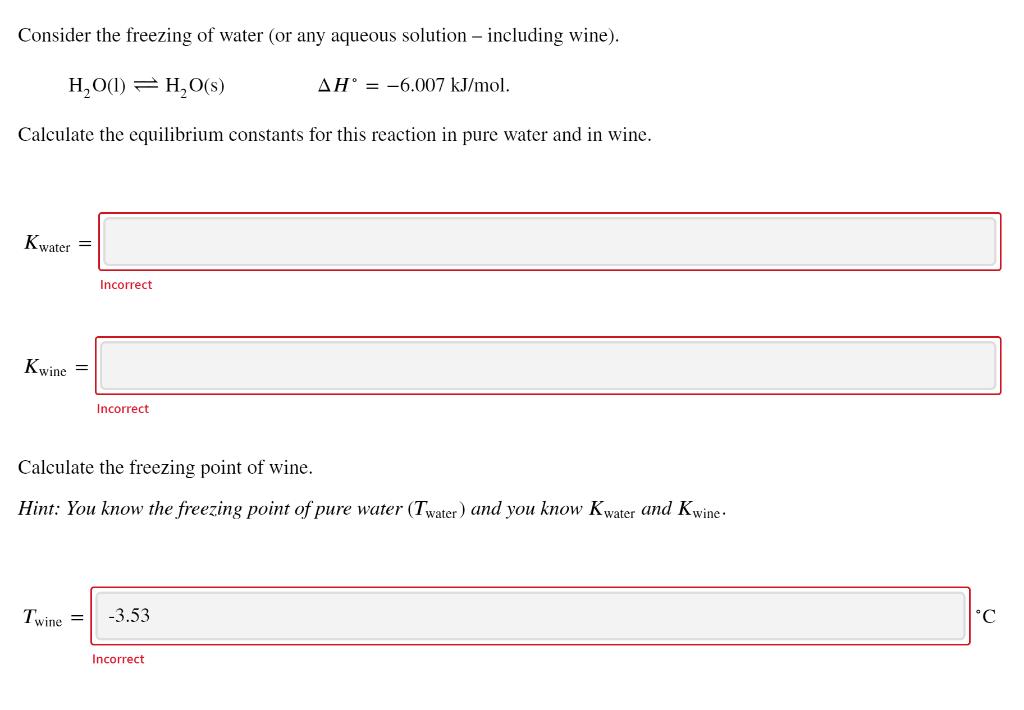
Consider the freezing of water (or any aqueous solution - including wine). H, O(1) = H,O(s) AH = -6.007 kJ/mol. Calculate the equilibrium constants for this reaction in pure water and in wine. Kwater = Incorrect Kwine = Incorrect Calculate the freezing point of wine. Hint: You know the freezing point of pure water (Twater) and you know Kwater and Kwine- Twine -3.53 Incorrect
Step by Step Solution
3.43 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics for Scientists and Engineers A Strategic Approach with Modern Physics
Authors: Randall D. Knight
4th edition
978-0134092508, 134092503, 133942651, 978-0133942651
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



