Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the H, molecule with atoms HA and Hg separated by a distance R: HA-Ha. The probability density for the 0y and o 1,orbitals
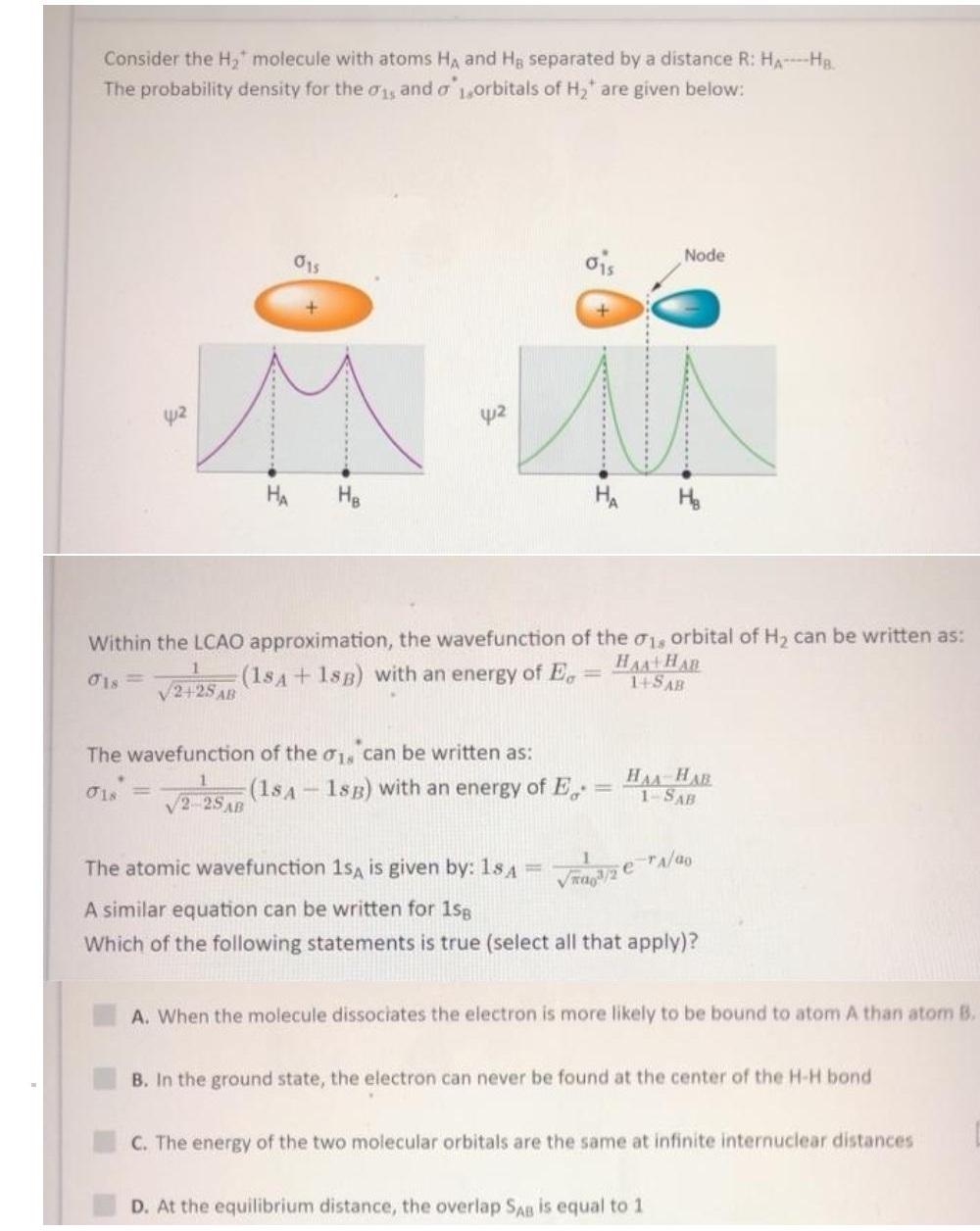
Consider the H, molecule with atoms HA and Hg separated by a distance R: HA-Ha. The probability density for the 0y and o 1,orbitals of H, are given below: ois Node 015 42 42 HA HA H. Within the LCAO approximation, the wavefunction of the 01, orbital of H, can be written as: (18A+ 1sB) with an energy of E, HAA+HAR 14SAB V2+25 AB The wavefunction of the o1, can be written as: (1sA - 1sB) with an energy of E, HAA HAR 1-SAB O1s = V2-25AB The atomic wavefunction 1sA is given by: 1s A %3D Vra/2 erA/ao A similar equation can be written for 1sg Which of the following statements is true (select all that apply)? A. When the molecule dissociates the electron is more likely to be bound to atom A than atom B. B. In the ground state, the electron can never be found at the center of the H-H bond C. The energy of the two molecular orbitals are the same at infinite internuclear distances D. At the equilibrium distance, the overlap SAB is equal to 1 .......
Step by Step Solution
★★★★★
3.43 Rating (140 Votes )
There are 3 Steps involved in it
Step: 1
Statement A FALSE Because the probability density function is symm...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


