Answered step by step
Verified Expert Solution
Question
1 Approved Answer
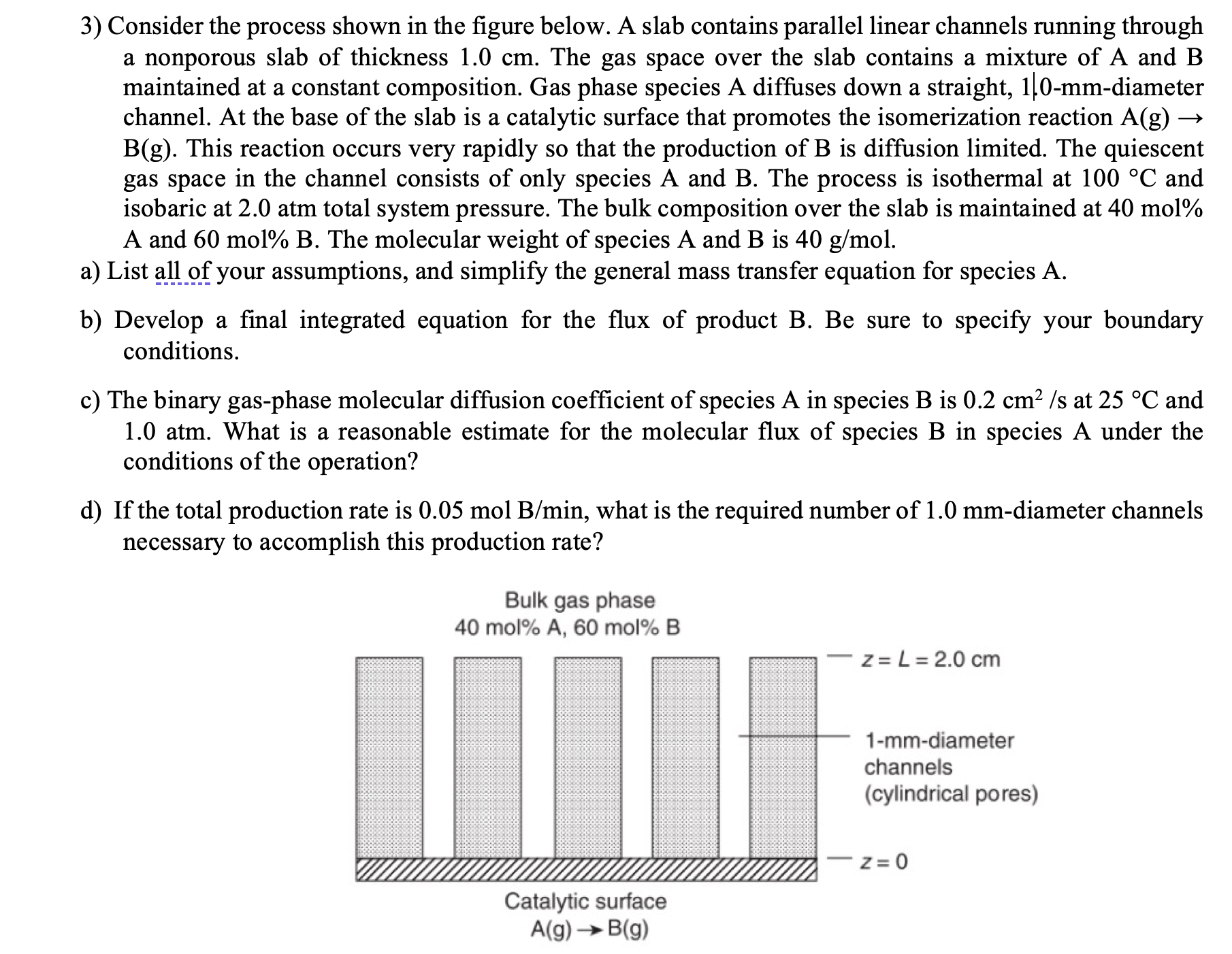
Consider the process shown in the figure below. A slab contains parallel linear channels running through a nonporous slab of thickness 1 . 0 c
Consider the process shown in the figure below. A slab contains parallel linear channels running through
a nonporous slab of thickness The gas space over the slab contains a mixture of A and
maintained at a constant composition. Gas phase species A diffuses down a straight, mmdiameter
channel. At the base of the slab is a catalytic surface that promotes the isomerization reaction
This reaction occurs very rapidly so that the production of is diffusion limited The quiescent
gas space in the channel consists of only species A and The process is isothermal at and
isobaric at atm total system pressure. The bulk composition over the slab is maintained at mol
A and mol The molecular weight of species A and B is
a List all of your assumptions, and simplify the general mass transfer equation for species
b Develop a final integrated equation for the flux of product B Be sure to specify your boundary
conditions.
c The binary gasphase molecular diffusion coefficient of species A in species B is at and
atm. What is a reasonable estimate for the molecular flux of species B in species A under the
conditions of the operation?
d If the total production rate is mol what is the required number of diameter channels
necessary to accomplish this production rate?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


