Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the production of 1,1-dichloroethane (C2H4Cl2) from ethylene (C2H4) and chlorine (Cl2). This gas-phase reaction is the first step in producing polyvinyl chloride (PVC). The
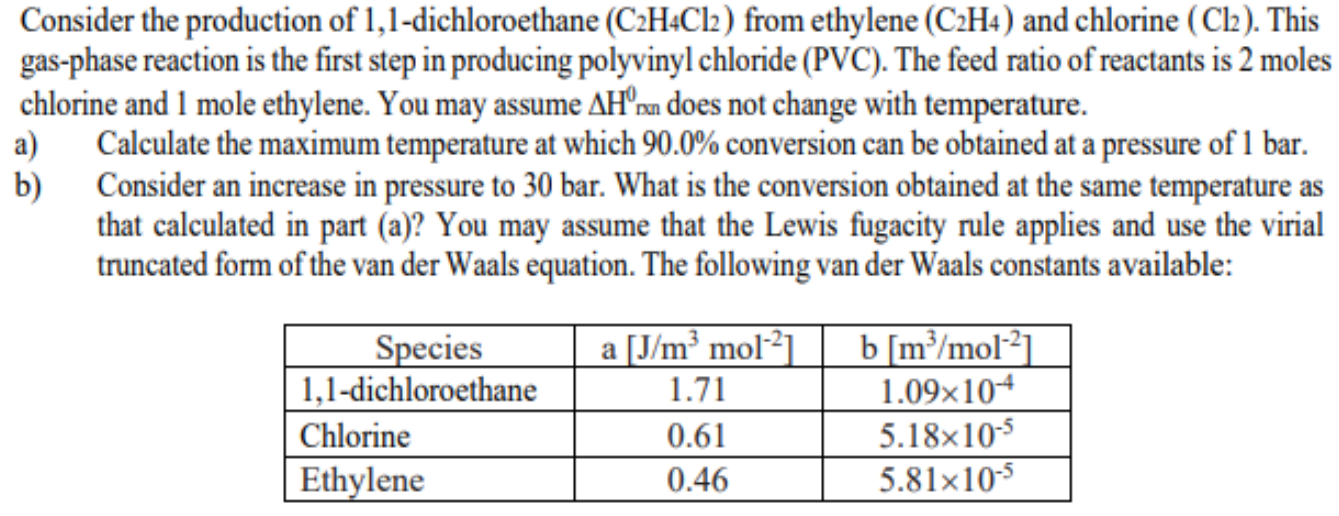 Consider the production of 1,1-dichloroethane (C2H4Cl2) from ethylene (C2H4) and chlorine (Cl2). This gas-phase reaction is the first step in producing polyvinyl chloride (PVC). The feed ratio of reactants is 2 moles chlorine and 1 mole ethylene. You may assume H0mnn does not change with temperature. a) Calculate the maximum temperature at which 90.0% conversion can be obtained at a pressure of 1 bar. b) Consider an increase in pressure to 30 bar. What is the conversion obtained at the same temperature as that calculated in part (a)? You may assume that the Lewis fugacity rule applies and use the virial truncated form of the van der Waals equation. The following van der Waals constants available
Consider the production of 1,1-dichloroethane (C2H4Cl2) from ethylene (C2H4) and chlorine (Cl2). This gas-phase reaction is the first step in producing polyvinyl chloride (PVC). The feed ratio of reactants is 2 moles chlorine and 1 mole ethylene. You may assume H0mnn does not change with temperature. a) Calculate the maximum temperature at which 90.0% conversion can be obtained at a pressure of 1 bar. b) Consider an increase in pressure to 30 bar. What is the conversion obtained at the same temperature as that calculated in part (a)? You may assume that the Lewis fugacity rule applies and use the virial truncated form of the van der Waals equation. The following van der Waals constants available Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


