Answered step by step
Verified Expert Solution
Question
1 Approved Answer
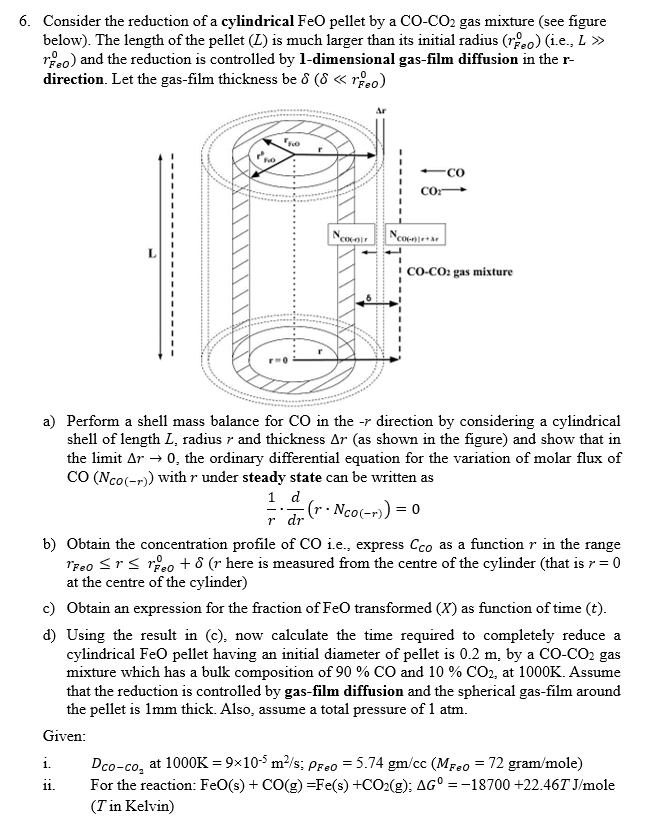
Consider the reduction of a cylindrical FeO pellet by a C O - C O 2 gas mixture ( see figure below ) . The
Consider the reduction of a cylindrical FeO pellet by a gas mixture see figure
below The length of the pellet is much larger than its initial radius ie
: and the reduction is controlled by dimensional gasfilm diffusion in the
direction. Let the gasfilm thickness be
a Perform a shell mass balance for in the direction by considering a cylindrical
shell of length radius and thickness as shown in the figure and show that in
the limit the ordinary differential equation for the variation of molar flux of
with under steady state can be written as
b Obtain the concentration profile of ie express as a function in the range
here is measured from the centre of the cylinder that is
at the centre of the cylinder
c Obtain an expression for the fraction of FeO transformed as function of time
d Using the result in c now calculate the time required to completely reduce a
cylindrical FeO pellet having an initial diameter of pellet is by a gas
mixture which has a bulk composition of and at Assume
that the reduction is controlled by gasfilm diffusion and the spherical gasfilm around
the pellet is thick. Also, assume a total pressure of atm.
Given:
i at ;graole
ii For the reaction: FeO;ole
in Kelvin
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


