Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the scheme of elementary reactions : k1=10e3500/T,sec1k2=1012e10500T,sec1k3=108e7000/T,sec1 Feed consists of A and inerts, CA0=1mole/ liter, and the operable temperature range is between 7 and
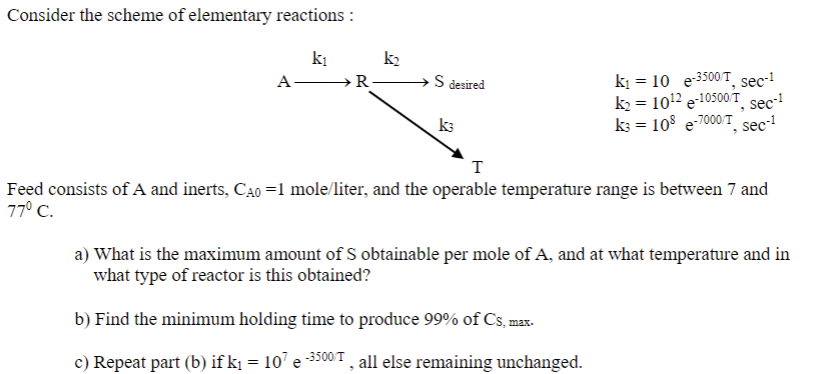 Consider the scheme of elementary reactions : k1=10e3500/T,sec1k2=1012e10500T,sec1k3=108e7000/T,sec1 Feed consists of A and inerts, CA0=1mole/ liter, and the operable temperature range is between 7 and 770C a) What is the maximum amount of S obtainable per mole of A, and at what temperature and in what type of reactor is this obtained? b) Find the minimum holding time to produce 99% of Cs, max. c) Repeat part (b) if k1=107e3500T, all else remaining unchanged
Consider the scheme of elementary reactions : k1=10e3500/T,sec1k2=1012e10500T,sec1k3=108e7000/T,sec1 Feed consists of A and inerts, CA0=1mole/ liter, and the operable temperature range is between 7 and 770C a) What is the maximum amount of S obtainable per mole of A, and at what temperature and in what type of reactor is this obtained? b) Find the minimum holding time to produce 99% of Cs, max. c) Repeat part (b) if k1=107e3500T, all else remaining unchanged Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


