Answered step by step
Verified Expert Solution
Question
1 Approved Answer
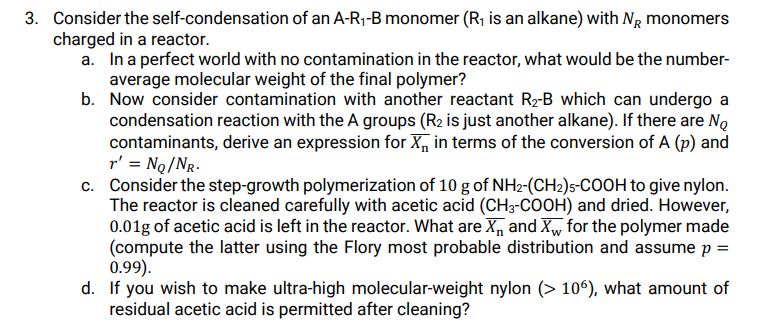
Consider the self - condensation of an A - R 1 - B monomer ( R 1 is an alkane ) with N R monomers
Consider the selfcondensation of an monomer is an alkane with monomers
charged in a reactor.
a In a perfect world with no contamination in the reactor, what would be the number
average molecular weight of the final polymer?
b Now consider contamination with another reactant which can undergo a
condensation reaction with the A groups is just another alkane If there are
contaminants, derive an expression for in terms of the conversion of and
c Consider the stepgrowth polymerization of of to give nylon.
The reactor is cleaned carefully with acetic acid and dried. However,
of acetic acid is left in the reactor. What are and for the polymer made
compute the latter using the Flory most probable distribution and assume
d If you wish to make ultrahigh molecularweight nylon what amount of
residual acetic acid is permitted after cleaning?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


