Question
Consider the table below and answer the questions that follow: Atomic Radius Crystal Structure (nm) 0.1246 0.071 Element Ni H Ag Cr Co FCC
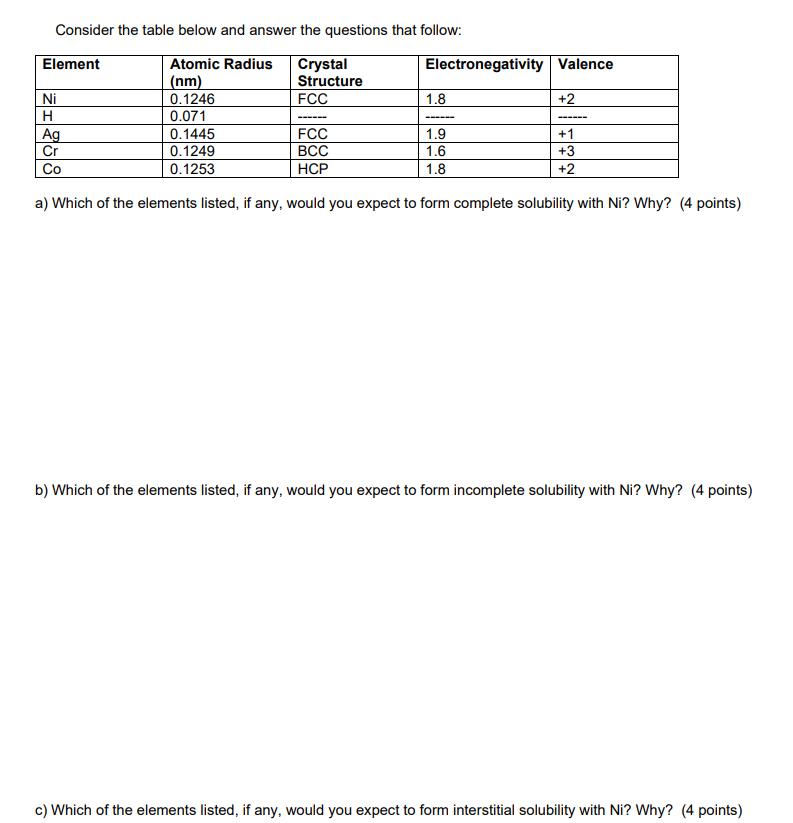
Consider the table below and answer the questions that follow: Atomic Radius Crystal Structure (nm) 0.1246 0.071 Element Ni H Ag Cr Co FCC 0.1445 0.1249 0.1253 ------ Electronegativity Valence 1.8 ---- +2 FCC 1.9 BCC 1.6 HCP 1.8 a) Which of the elements listed, if any, would you expect to form complete solubility with Ni? Why? (4 points) ------ +1 +3 +2 b) Which of the elements listed, if any, would you expect to form incomplete solubility with Ni? Why? (4 points) c) Which of the elements listed, if any, would you expect to form interstitial solubility with Ni? Why? (4 points)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Sure Here is my analysis of the table a Complete solubility Ni and Cr Both have the same crystal structure FCC and their atomic radii are similar 0124...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Materials Science and Engineering An Introduction
Authors: William D. Callister Jr., David G. Rethwisch
9th edition
978-1118546895, 111854689X, 978-1118477700, 1118477707, 1118324579, 978-1118324578
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



