Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the titration described here: 0.7917 g of anhydrous sodium thiosulphate (Na 2 S 2 O 3 ), previously purified and dried in an oven,
Consider the titration described here:
- 0.7917 g of anhydrous sodium thiosulphate (Na 2 S 2 O 3 ), previously purified and dried in an oven, is weighed into an erlenmeyer flask.
- This sample is titrated with a mixture of KIO 3 and KI that contains approximately 0.1 M I 3 - . This titration requires 23.56 mL of titrant to reach the endpoint (found with a starch indicator).
- This same mixture of KIO 3 and Kl is then used to titrate a solution containing an unknown amount of ascorbic acid. This titration requires 37.21 mL of titrant to reach the endpoint (found with a starch indicator).
- The ascorbic acid sample used in the titration above was prepared by using a 25 mL pipet to deliver an aliquot of the solution into an erlenmeyer flask.
Relevant reactions:
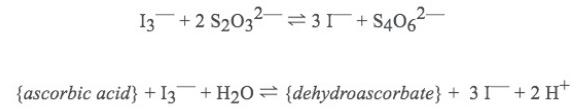
Based on this information, what is the concentration (in mol/L) of ascorbic acid in the "unknown" liquid?
13 +2 S20331 +S406 {ascorbic acid} +13 + HO = {dehydroascorbate} + 31 + 2H+
Step by Step Solution
★★★★★
3.52 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
ANSWER In this titration iodine is generated by the reaction of potassium iodate KIO3 and potassium ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


