Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the van der Waals model for a non-ideal gas. The improved model approximates the behavior of real fluids by considering the intermolecular attraction
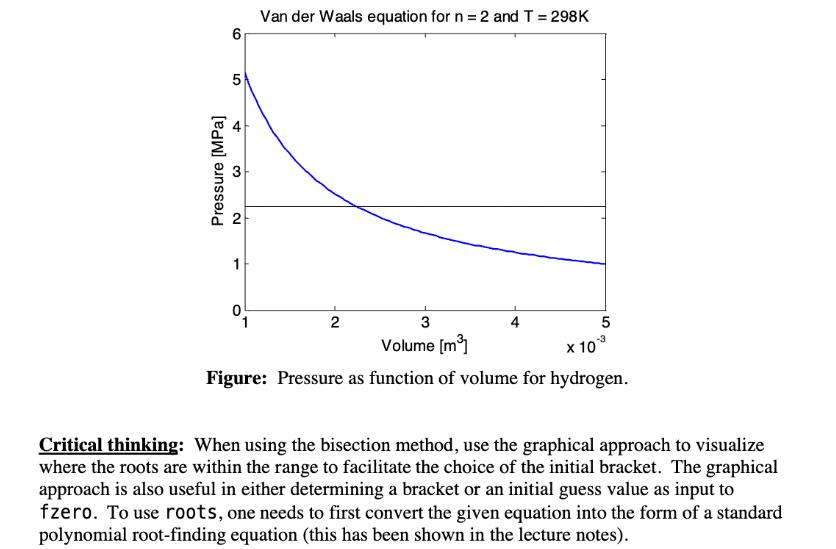
Consider the van der Waals model for a non-ideal gas. The improved model approximates the behavior of real fluids by considering the intermolecular attraction force between molecules (factor a) and their nonzero sizes (factor b). (P + ) (V - nb) = nRT where R 8.314 J/mole-K is the universal gas constant. Consider two moles (n = 2) of hydrogen at a fixed temperature T = 298 K (25 C). The material constants in the equation are: a = 0.0245 J. m/mole and b = 2.66 10-5 m/mole. By rearranging the equation, one can plot the pressure as a function of volume. The figure below plots P versus V for 10- 6 5 Van der Waals equation for n = 2 and T = 298K st Pressure [MPa] 2 1 2 3 4 5 Volume [m] -3 x 10 Figure: Pressure as function of volume for hydrogen. Critical thinking: When using the bisection method, use the graphical approach to visualize where the roots are within the range to facilitate the choice of the initial bracket. The graphical approach is also useful in either determining a bracket or an initial guess value as input to fzero. To use roots, one needs to first convert the given equation into the form of a standard polynomial root-finding equation (this has been shown in the lecture notes).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


