Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider this dimensionless expression for the energy in terms of the velocity: (v) = (1 )-1/2 Note that in class, we worked with K(),
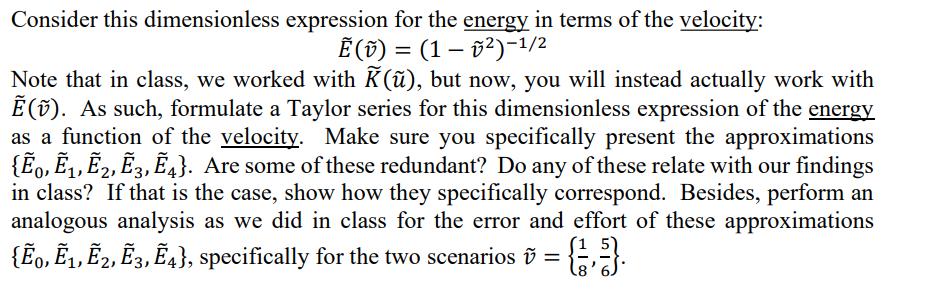
Consider this dimensionless expression for the energy in terms of the velocity: (v) = (1 )-1/2 Note that in class, we worked with K(), but now, you will instead actually work with (). As such, formulate a Taylor series for this dimensionless expression of the energy as a function of the velocity. Make sure you specifically present the approximations {0, E, E2, E3, 4}. Are some of these redundant? Do any of these relate with our findings in class? If that is the case, show how they specifically correspond. Besides, perform an analogous analysis as we did in class for the error and effort of these approximations {0, 2, E3, 4}, specifically for the two scenarios = {1}.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The Taylor series expansion youve provided centered at v 0 is as follows Ev E0 E1v E2v2 E3v3 E4v4 wh...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


