Question
Consider two boxes, each of which is filled with ideal gases. Consider that the two boxes are combined. 1. Under what condition or conditions
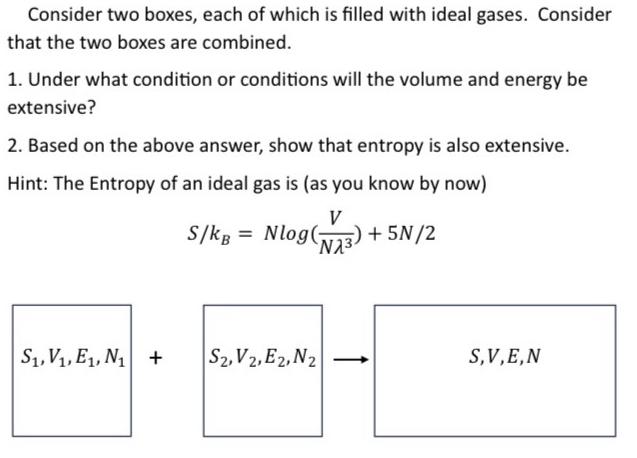
Consider two boxes, each of which is filled with ideal gases. Consider that the two boxes are combined. 1. Under what condition or conditions will the volume and energy be extensive? 2. Based on the above answer, show that entropy is also extensive. Hint: The Entropy of an ideal gas is (as you know by now) V NA +5N/2 S/kg Nlog(; = anaa + aran-[ S, V, E, N S2, V2, E2, N2 S,V,E,N
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Contemporary Auditing
Authors: Michael C Knapp
12th Edition
357515404, 978-0357515402
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



